Water

Overview
1. Physical properties.2. Polarity.
3. Solubility.
4. Hydrogen bond.
5. Hydrophobic effect.
6. Hydrophobic effect and Membranes.
7. Osmosis.(coming soon)
Intro
Extremeophile - Extremophiles are microorganisms (bacteria and archaea) that are adapted to survive extreme conditions
Psychrophiles near freezing waters (0-10C)
Thermophiles - in hot waters
Acidophiles - at low pH in acidic environments
Halophiles - in high salts
1. Physical properties
Water is life!
Unique properties of water make it essential to life:
Heat capacity is a measure of the amount of heat that is required to be applied to matter produce a unit change in the matter temperature.
Water high heat capacity means that water can absorb a lot heat before changing its temperature. That means that water serves as a "buffer" for heat.
Heat of vaporization is a measure of the amount of heat that is required to transform a substance from liquid to gas. Water high heat of vaporizations means that water can absorb a lot of heat before turning into vapor. In biological perspective this means that when water vaporizes from the skin it takes away a lot of heat. Therefore, water is very good at colling.
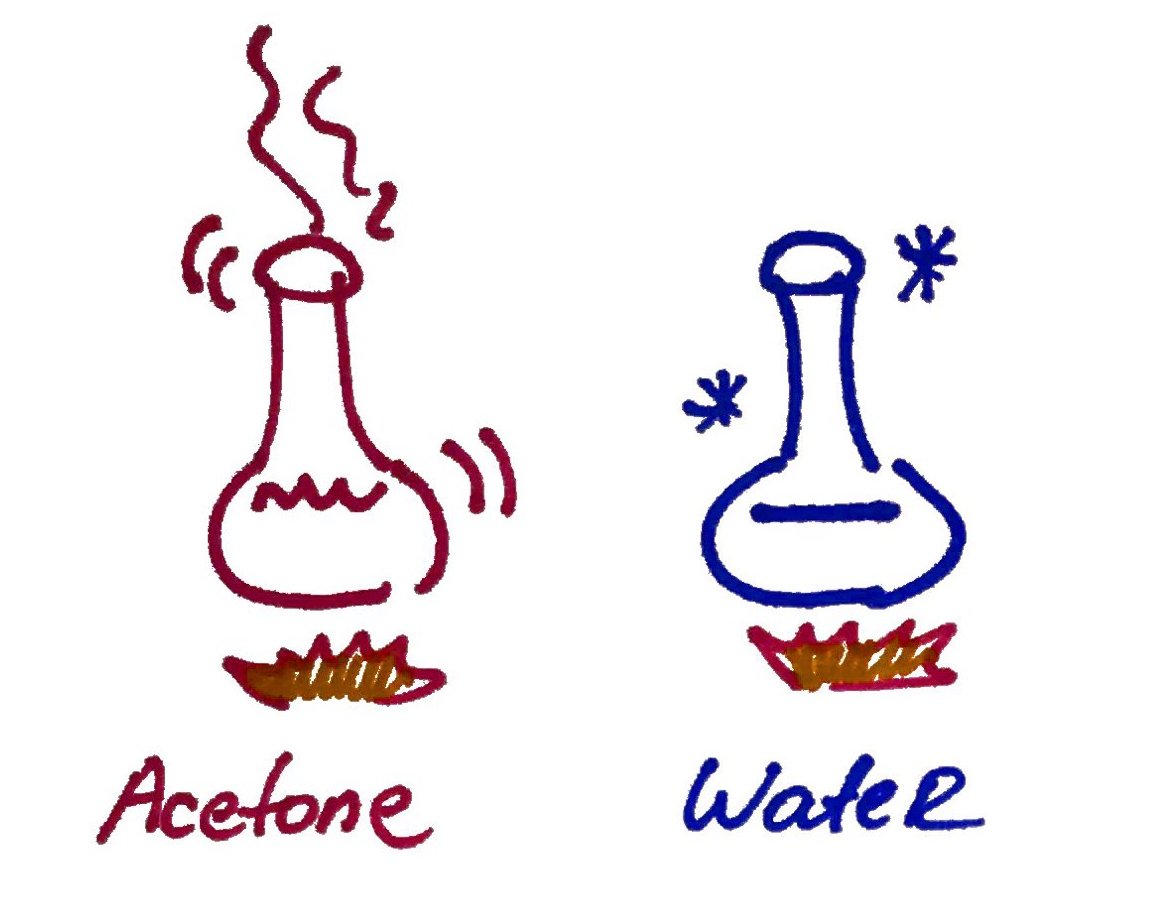
Remember it takes a lot of energy to heat up and vaporize water.
2. Polarity
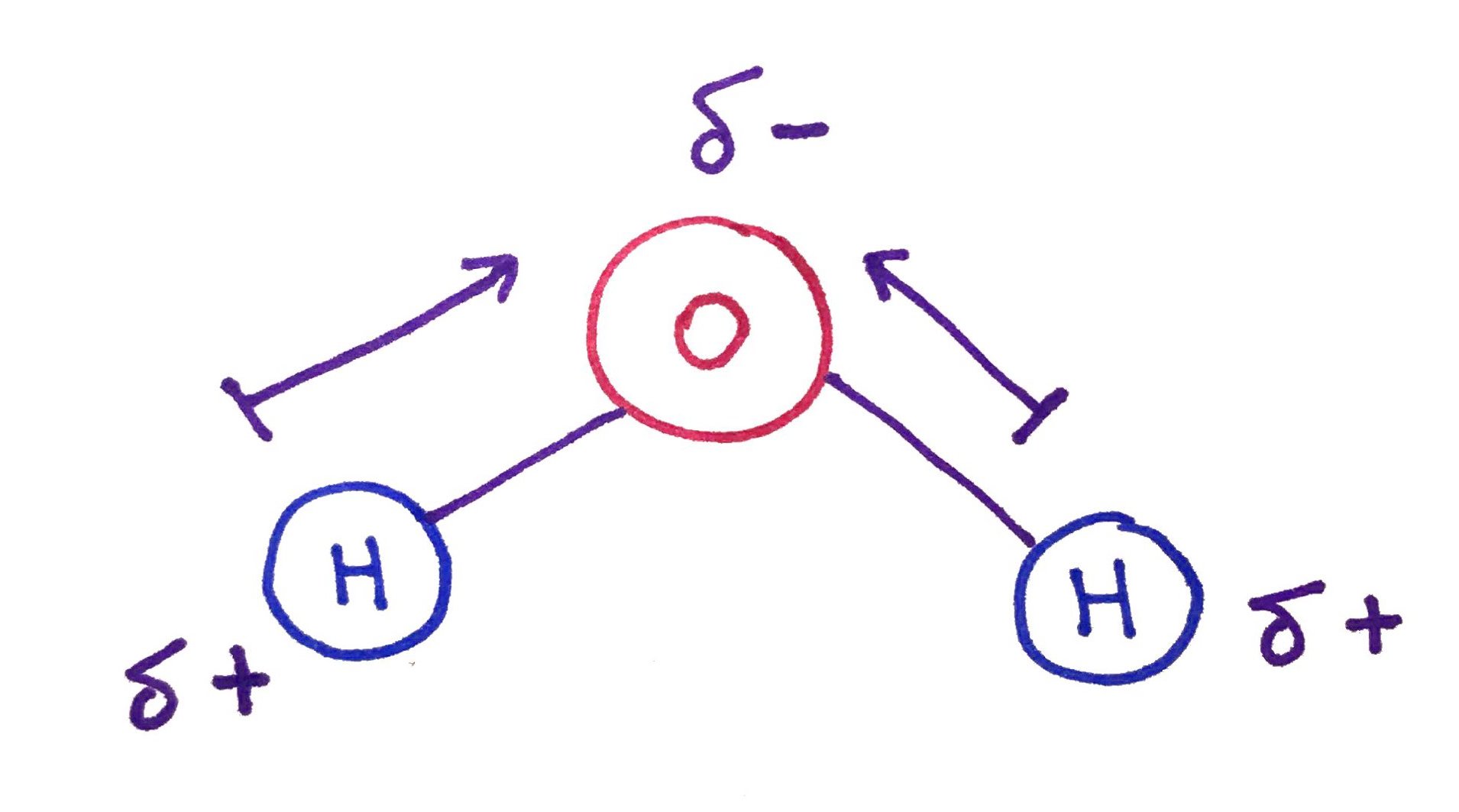
Water molecule is polar.
Oxygen atom is highly electronegative negative while the Hydrogen atom is less electronegative. Because of this electro-negativity difference Oxygen pulls the electron cloud onto itself. This uneven electron distribution creates a dipole or partial negative charge on the oxygen and partial positive charge on the hydrogen.
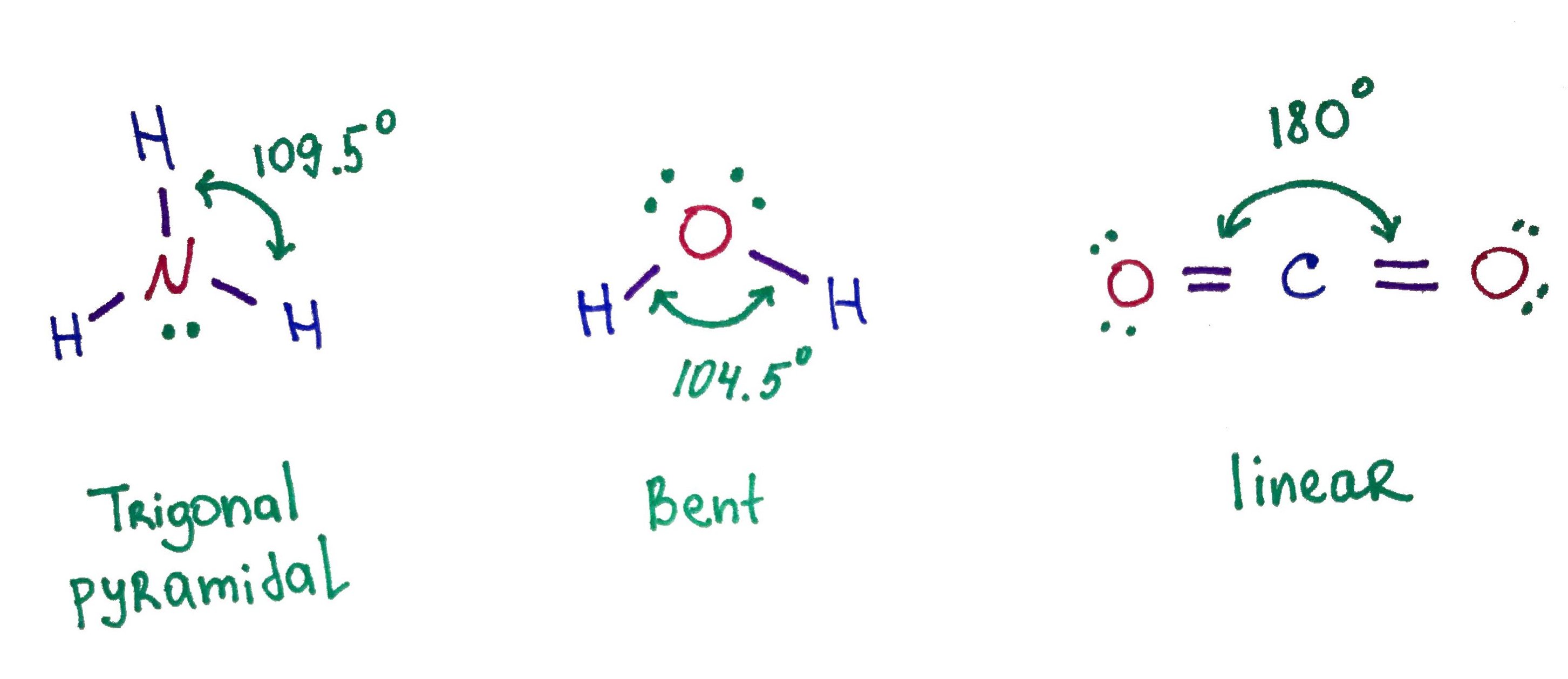
Water molecule polarity is also the result of its bent geometry. Ammonia is similar to water because it also has a dipole due to it's geometry. Carbon dioxide on the other hand is a linear molecule and it's dipole cancels out.
3. Solubility
Because of the water dipole it can solvate ionic bonds very well:
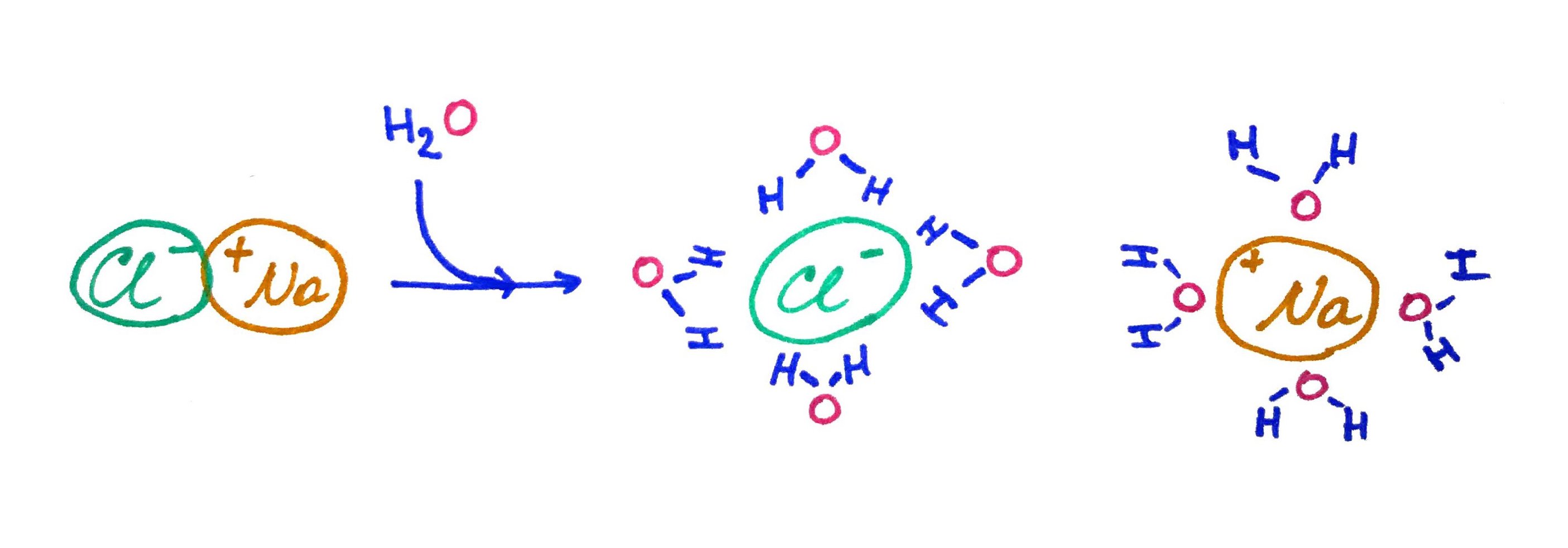
In this example Na+ and Cl- are bonded together by ionic bond. When we add water it solvates the ionic bond but it does not removes it completely. There is still some attraction but not as strong as it was before. In general we can measure the force of attraction between ions with Coulomb's Law:
Where F - force of attraction or repulsion, you have to use the common sense when assign the sign for the force. Remember that opposite charges attract (positive sign) while like charges repel (negative sign).
Charges q1 and q2 are magnitude charges, for NaCl it is 1 and 1.
Distance r^2 is how far apart are the charged particles.
Finlay ε dielectric constant most important constant, represents how well a substance can separate or "shield" charged particles from each other.
It is different for different substance for example water(polar molecule) is 80 and benzene(non polar molecule) is 4.
4. Hydrogen bond
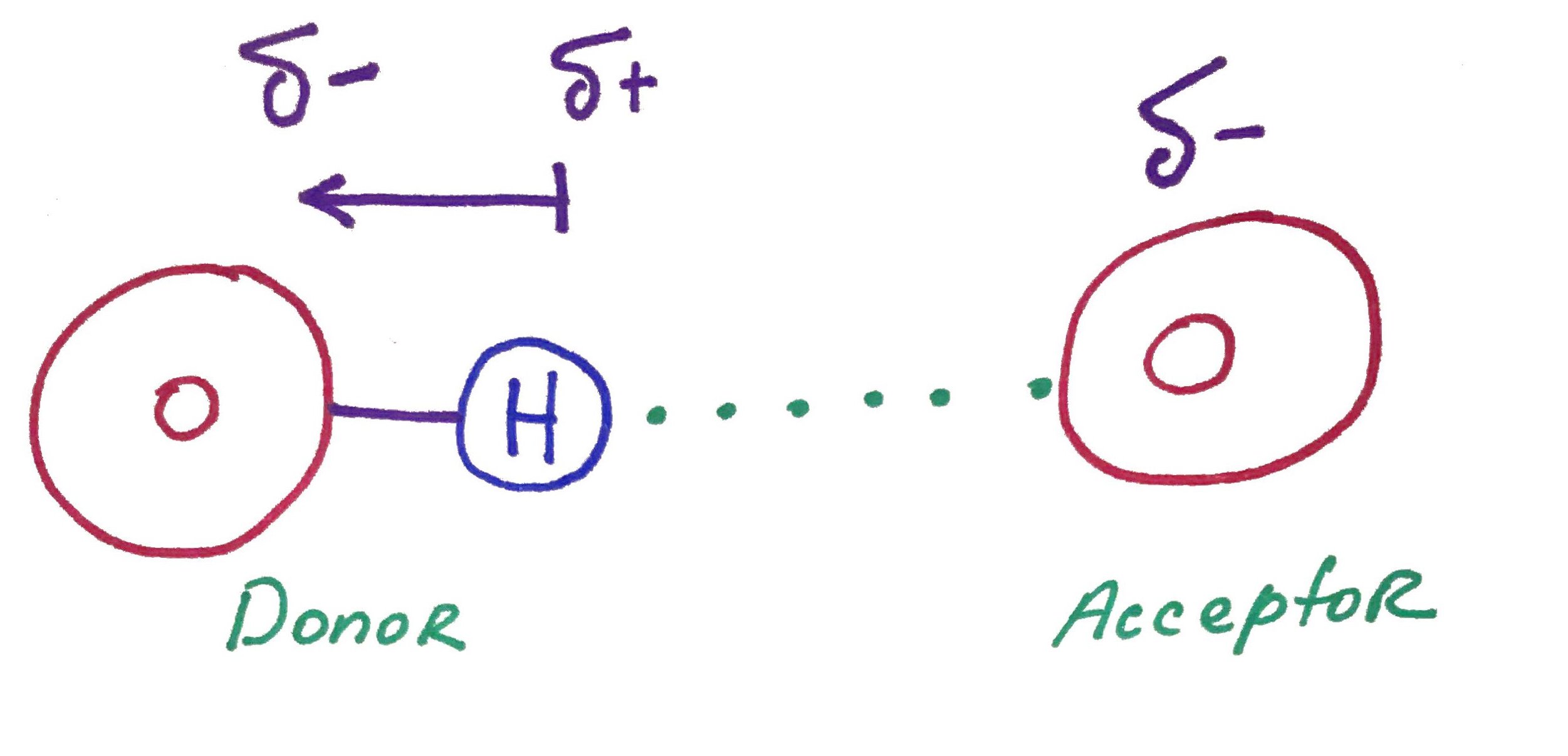
The true power of water is in it's ability to form hydrogen bonds.
Remember that hydrogen bonds are formed between a Donor, the molecule that has hydrogen covalently attached to itself, and an Acceptor, the molecule that does not have the hydrogen.
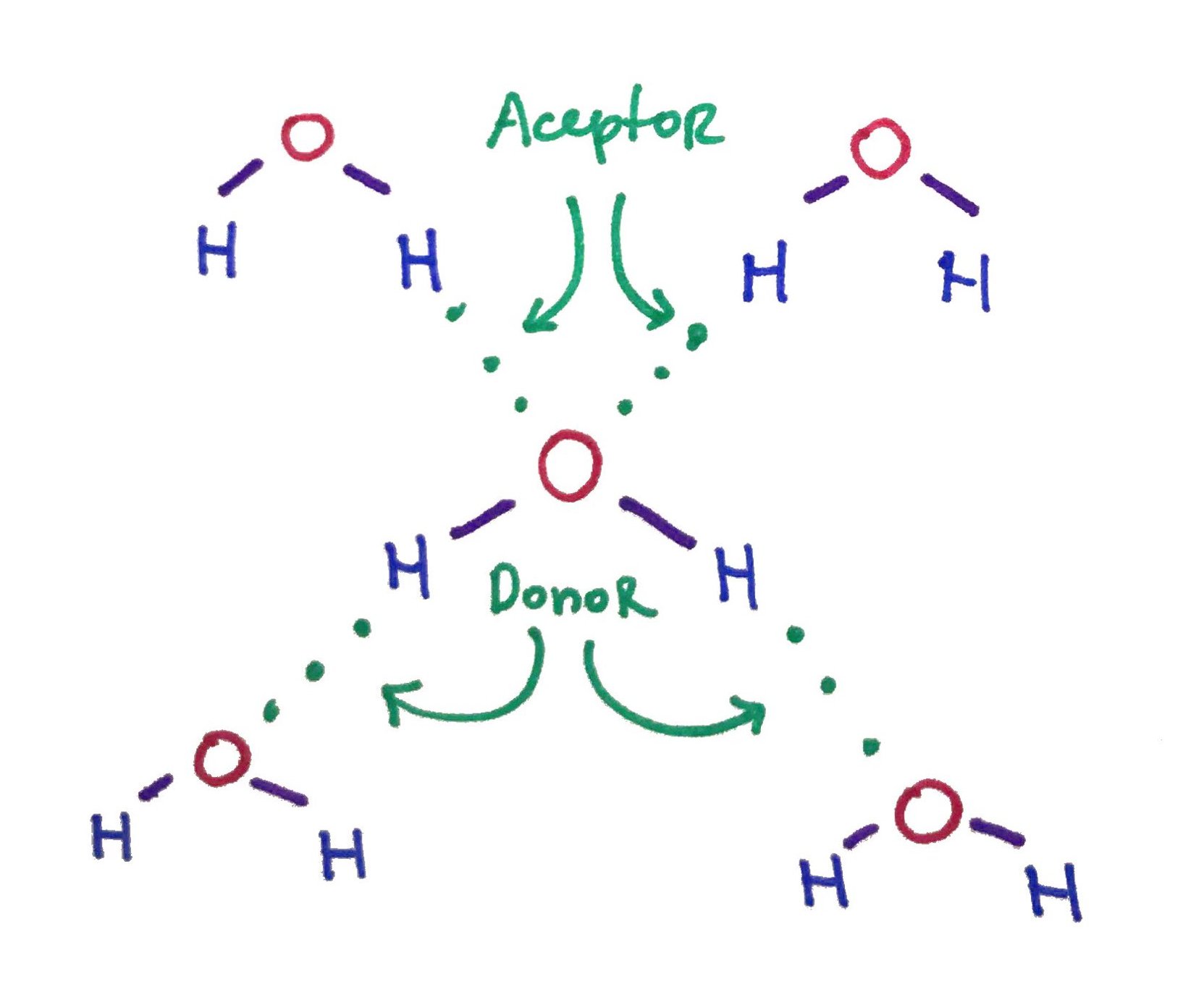
A single water molecule can form 4 Hydrogen bonds! 2 as a Donor and 2 as an Acceptor.
Remember that hydrogen bonds are a type of intermolecular forces (the interaction between molecules) as opposed to intramolecular forces (interactions within a molecule).
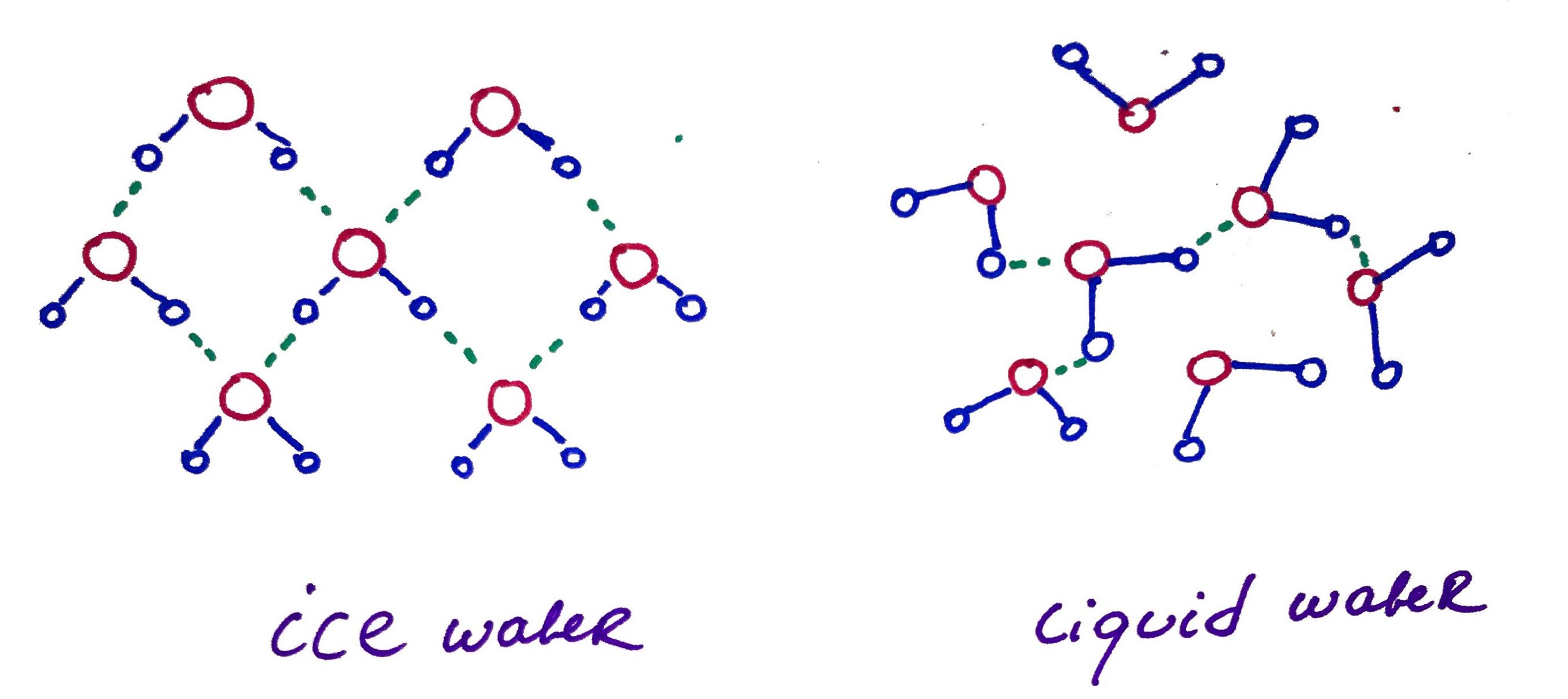
When water freeze each single water molecule establish 4 hydrogen bonds with 4 other molecules of water, forming crystal lattice that expands in space. This is why ice floats.
5. Hydrophobic effect
Depending on weather other molecules can form hydrogen bonds with water or not we can classify them as hydrophilic (water loving) or hydrophobic (afraid of water).
When hydrophobic molecule is placed in water they cannot hydrogen bond with each other, the only interactions that are available is London Dispersion Force also known as induced dipole-dipole interaction, which is one of the weakest interactions. This makes water molecules around the hydrophobic molecules to "freeze" in a cage or clathrate.
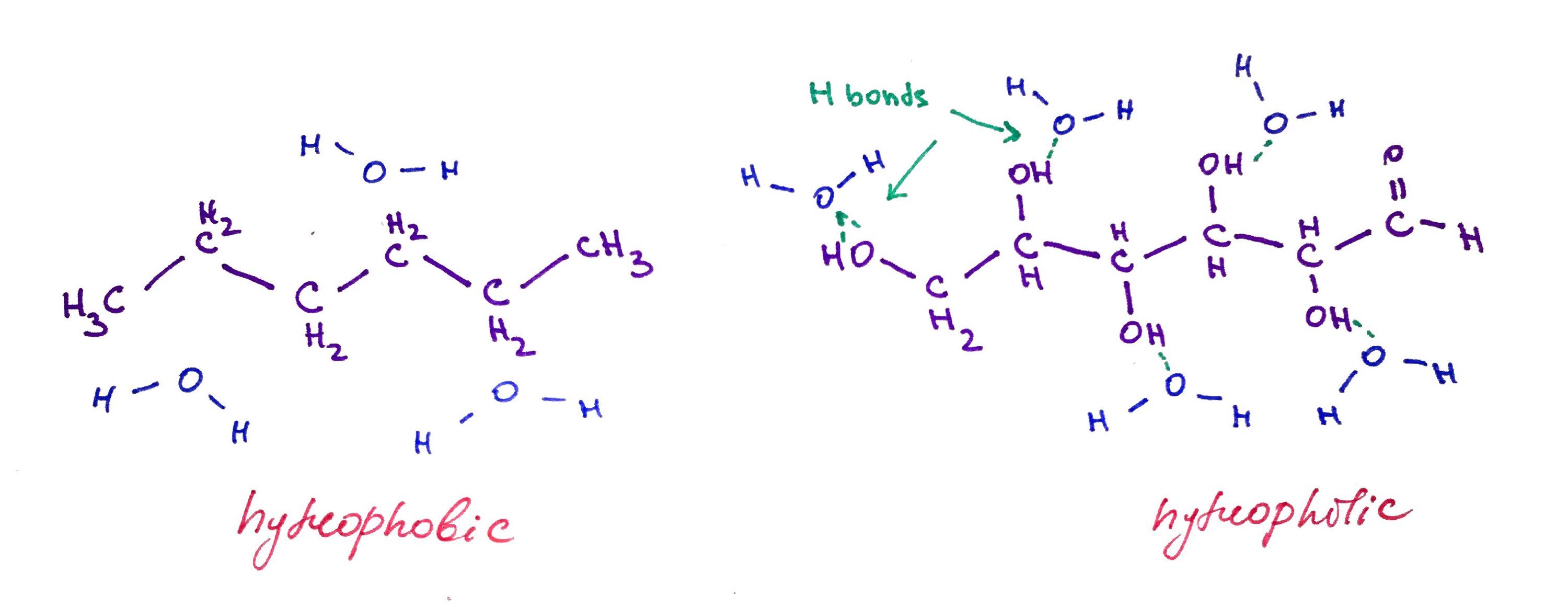
Hexane (6 carbon alkane is hydrophobic because it cannot hydrogen bond with water, while glucose (also 6 carbon molecule) has a lot of alcohol groups that allows it form hydrogen bonds with water.
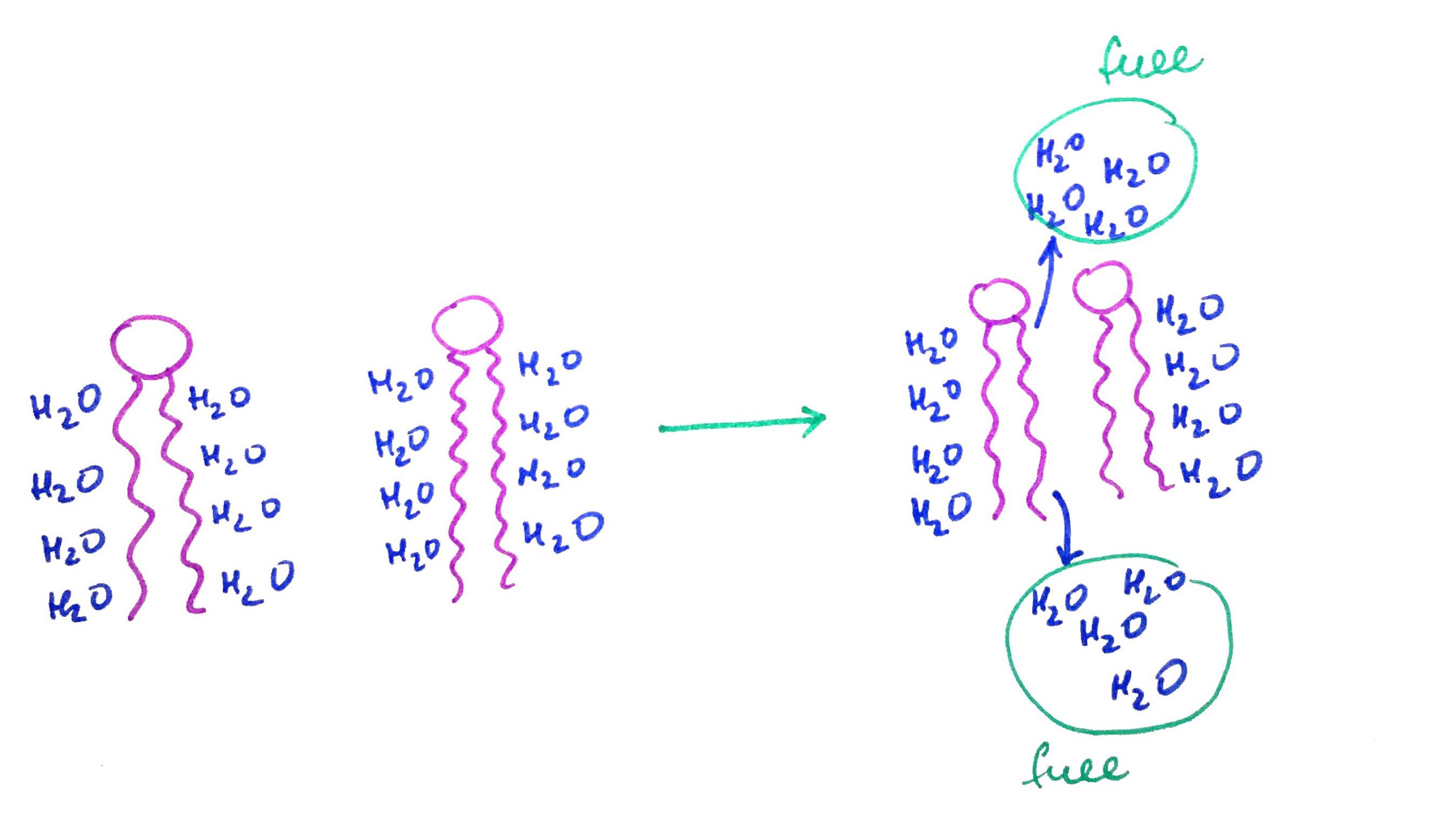
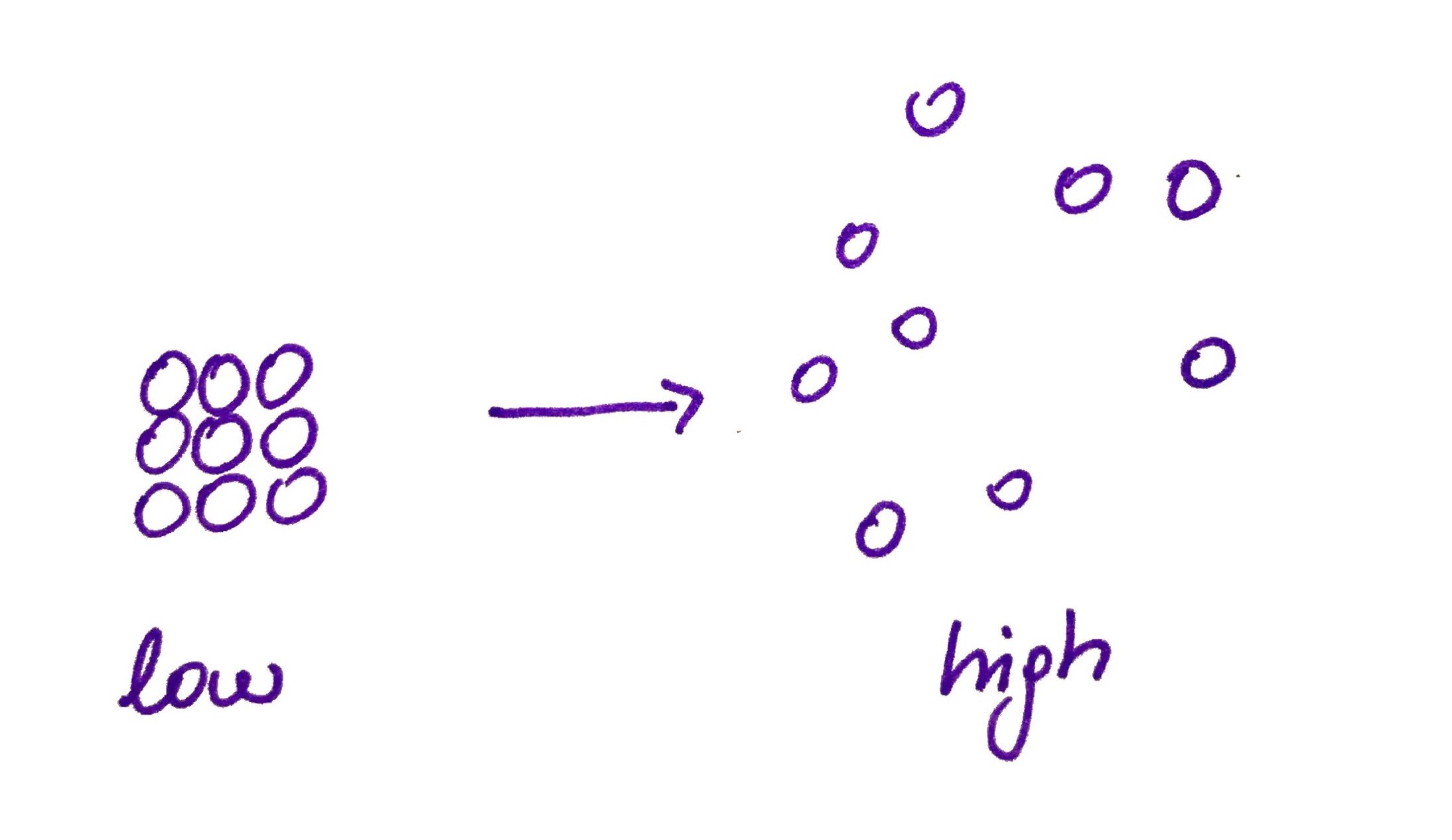
Water chlatrates have lower entropy therefore it is not favorite to form them (Remember entropy is always increases). Hydrophobic effect is the observed tendency of hydrophobic substances to aggregate in water to free up water molecules that are in clathrates.
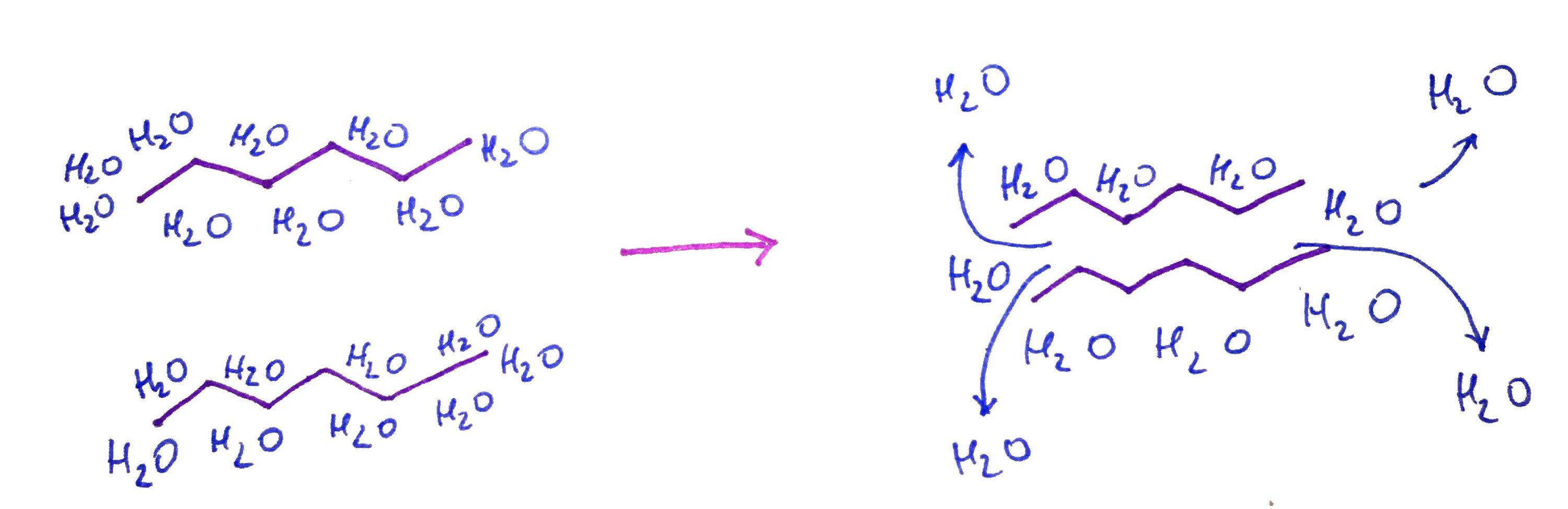
When submerged in water two Hexane moleculs will aggregate together freeing up water molecules from chlatrates in the process
6. Hydrophobic effect and Membranes
Hydrophobic effect is especially important in context of phospholipids and membranes. When placed in water phospholipids aggregate forming membranes.