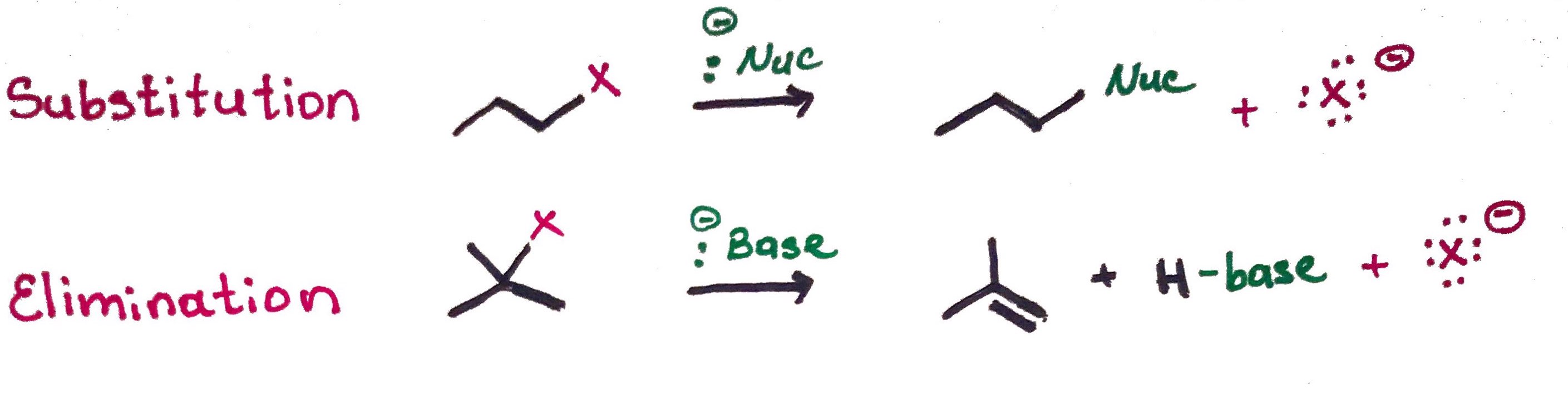
Substitution and Elimination of Alkyl halides
Overview
1. Intro.1. Intro
Where x = Cl, Br, I
- Halogen withdraws electron density via induction rendering C electrophilic
- Halogen serves as LG
- Alkyl halides classified into 1°, 2°, 3°
- Solvent effect:
1) Since SN2 Nuc is generally ionic, polar solvent is required.
2) Polar solvent, solvates transition state.
2. SN2 Reactions
Summary
SN2 - Substitution nucleophilic bimolecular.
Concerted mechanism, change in configuration, due to back side attack.
Nuc attack and loss of LG
Rate = k[alkyl halide][nucleophile]
3° < 2° < 1° < CH3 at alpha position due to steric hindrance
Strong Nuc: I-,Br-,Cl-, HS-, RS-, OH-, RO-, NC-
Polar Aprotic Solvent: Acetone, DMF, DMSO, Acetonitrile, HMPA
3. E2 Reactions
E2 - Elimination bimolecular. Also known as beta elimination or 1,2 - elimination.
Summary
Concerted mechanism
Proton transfer and loss of LG
Rate = k[alkyl halide][base]
1° < 2° < 3°
Strong Base: NaH, DBN, DBU, OH-, MeO-, EtO-
Stability of alkenes:
1. trans < cis
2. mono < di < tri < tetra substitutions
3. 3 < 4 < 5 < 6 > 7 member rings
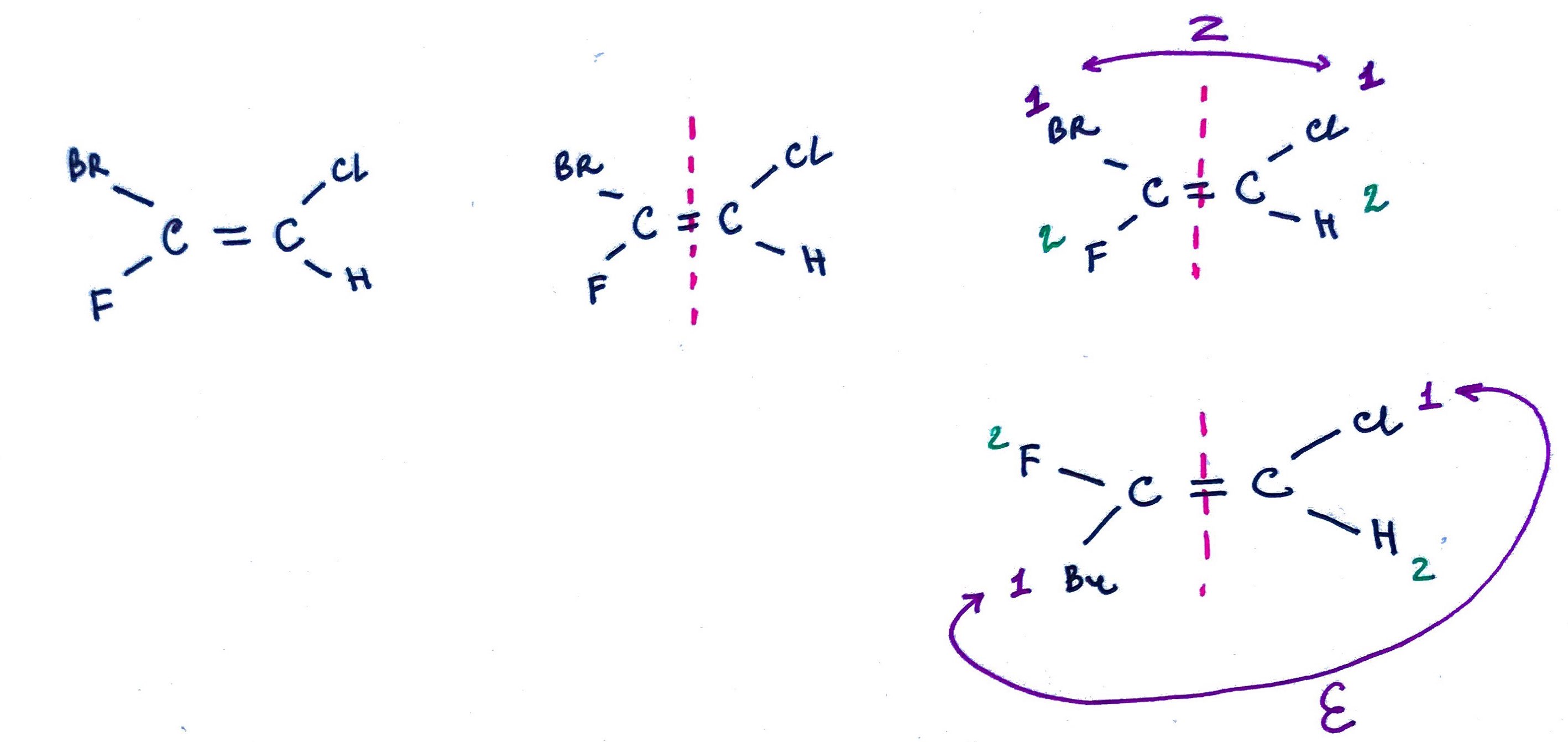
Z and E naming system
Sometimes it is hard to assign trans/cis configuration so the E/Z system is used instead:
1. Split the double bond in the middle with 1 carbon on each side.
2. Assign atom priorities based on Chan-Prelog-Ingold rules.
3. Z = same, E = opposite highest priorities.
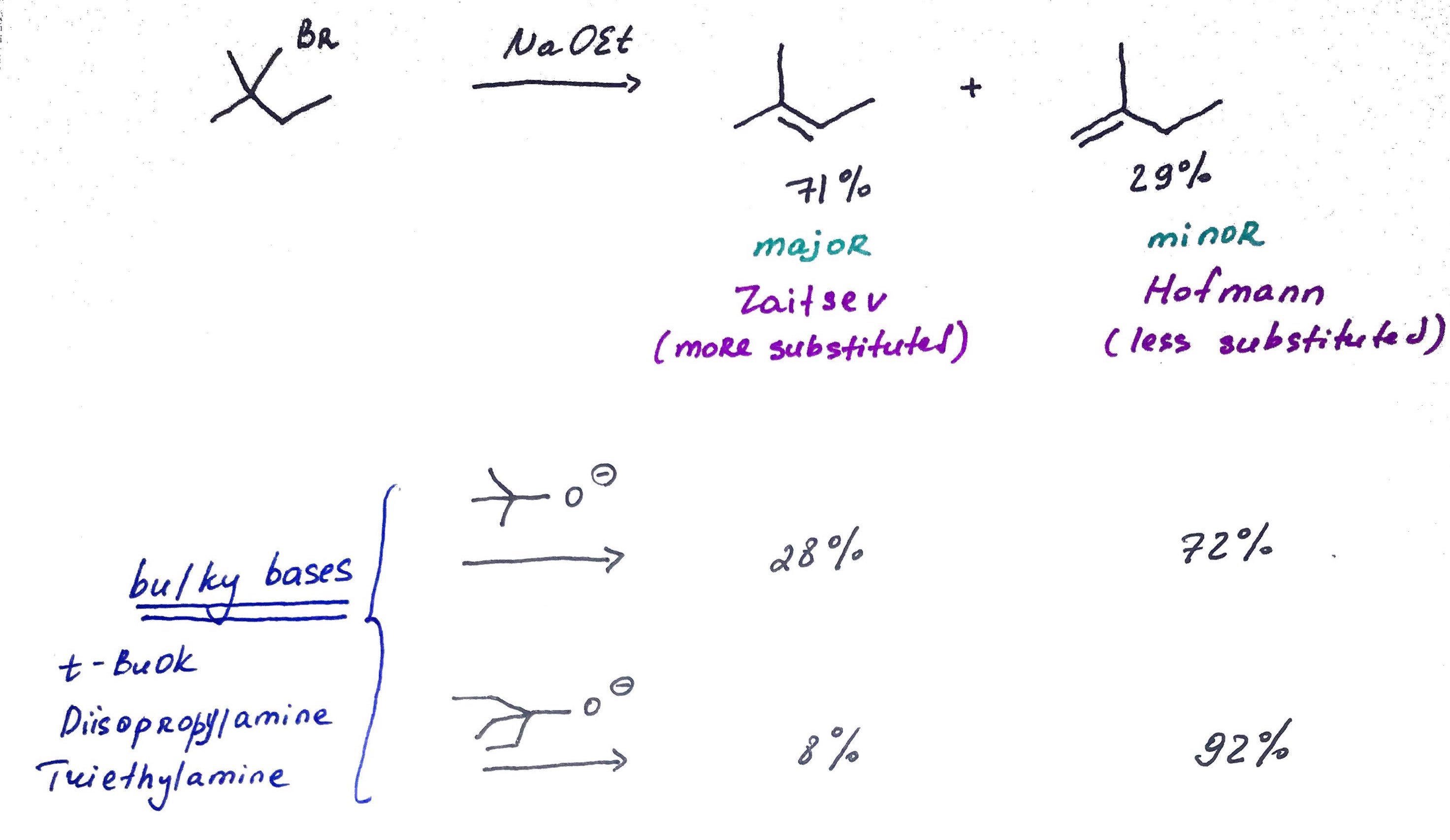
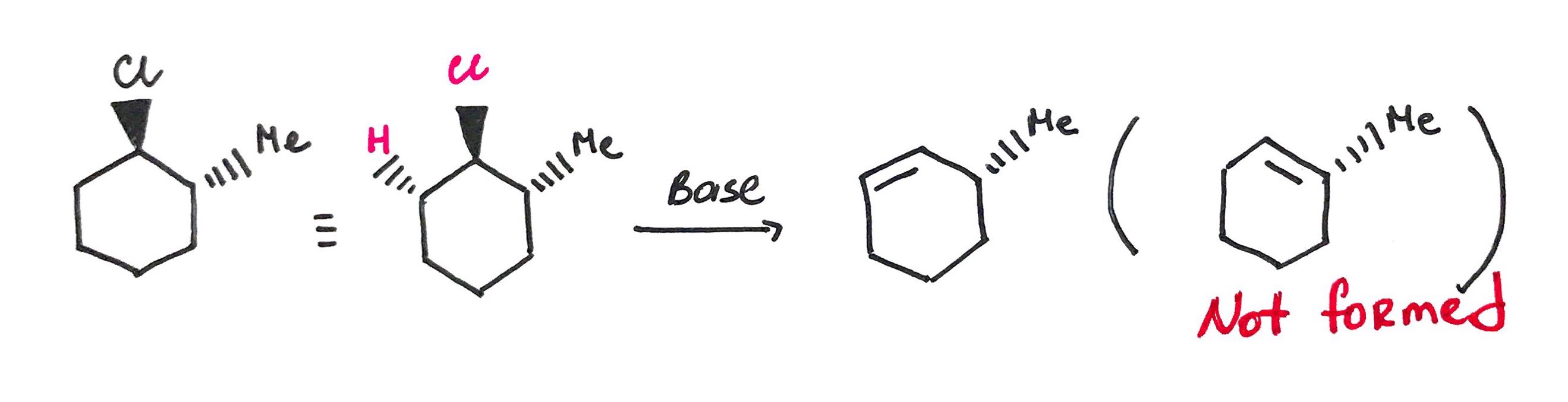
substrate is not necessarily sterioisomeric, but it can produce two sterioisomeric products that will be major and minor.
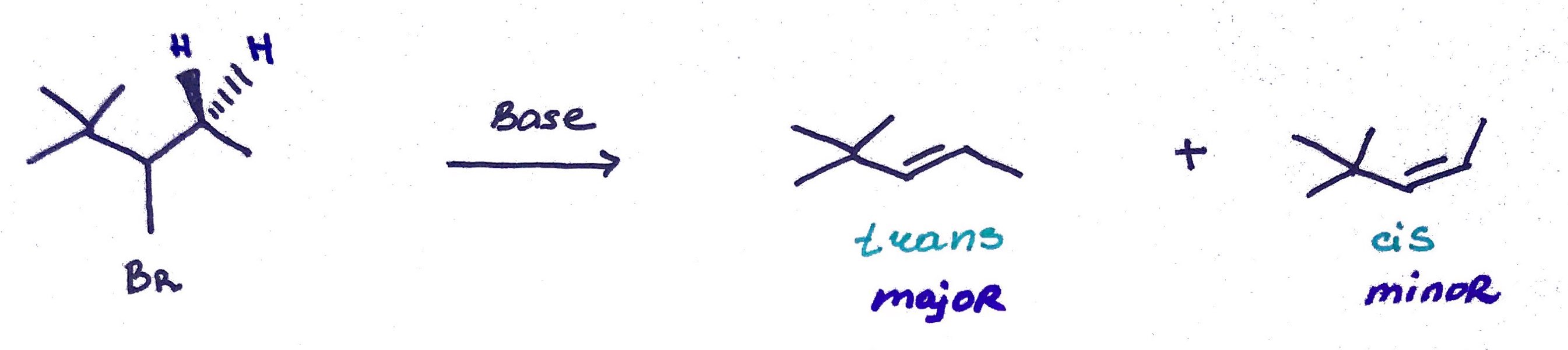
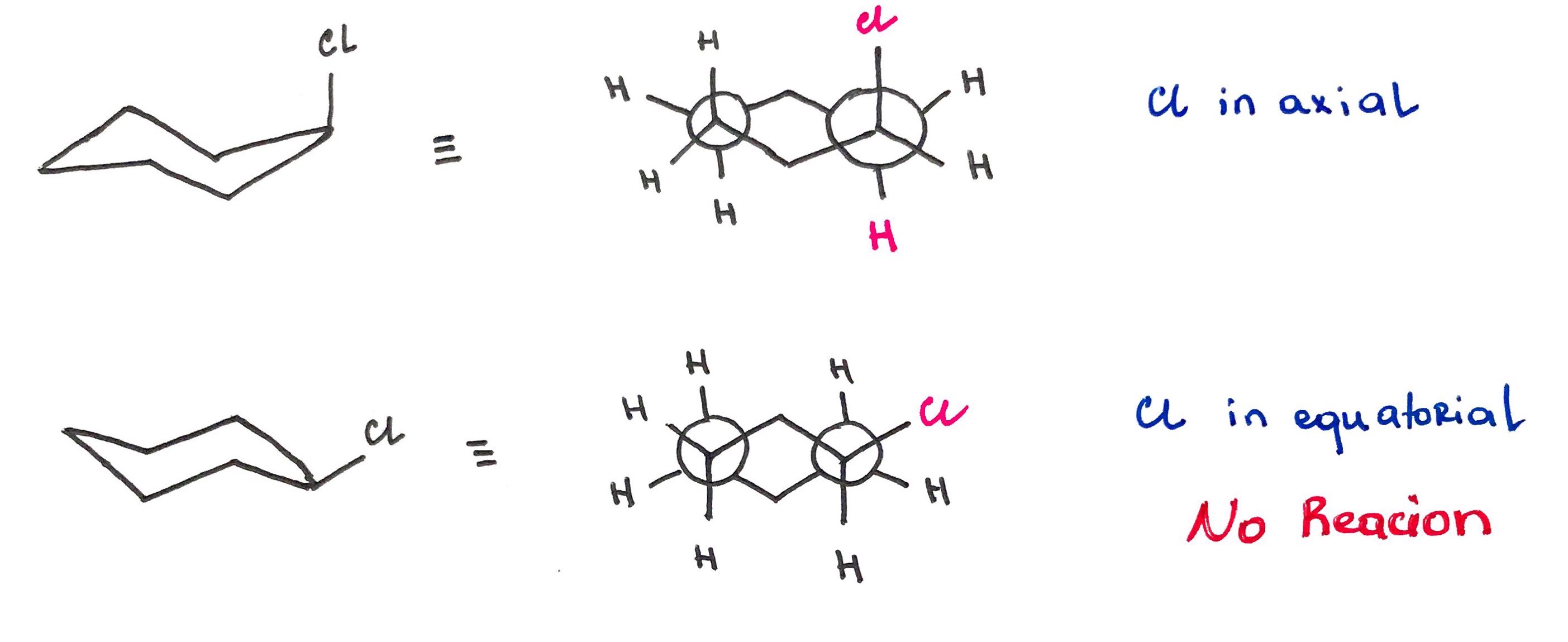
For the elimination to occur the halogen and proton must lie in coplanar plane (i.e dihedral angle must be 180°) OR periplanar plane (i.e dihedral angle must be close to 180°)
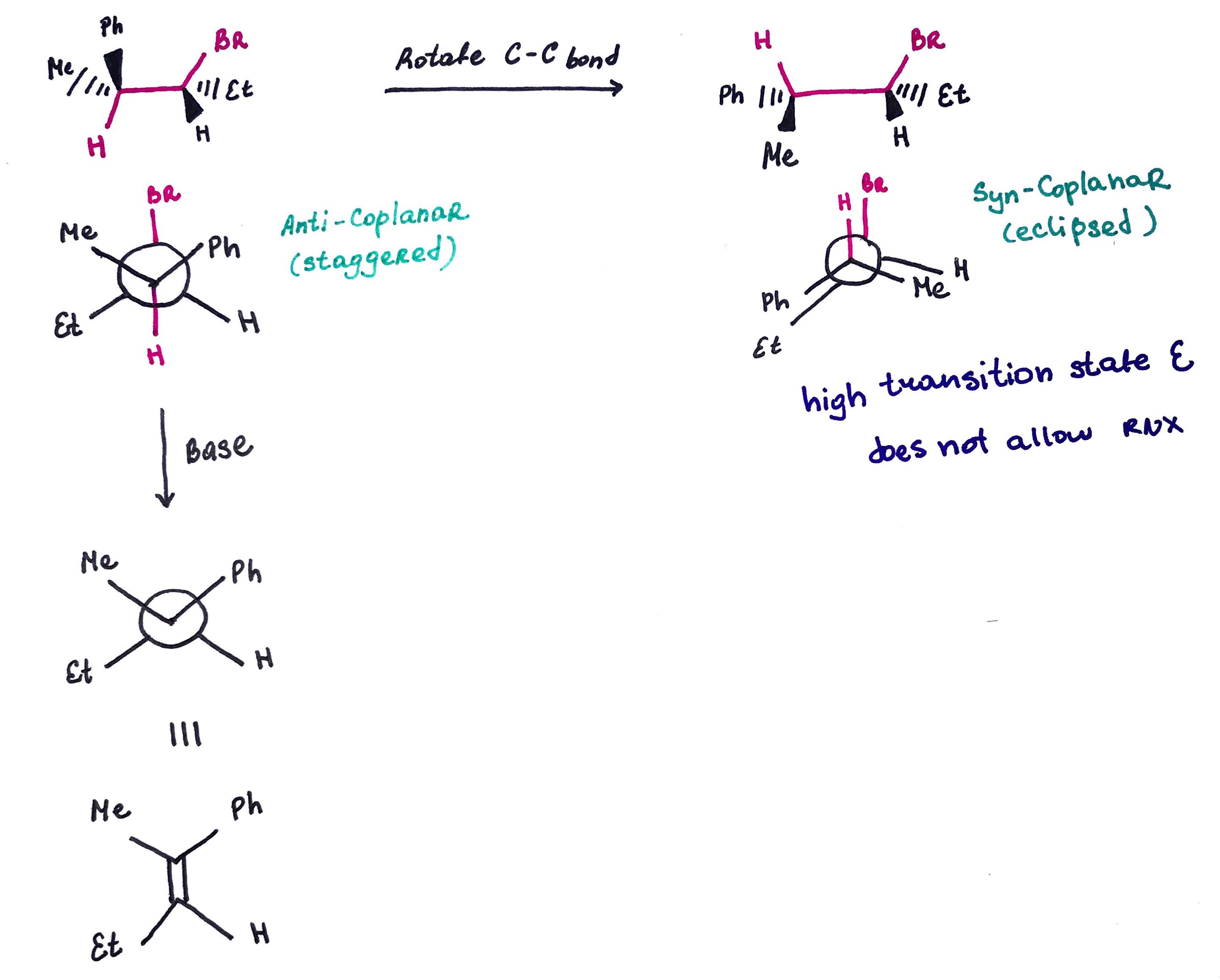
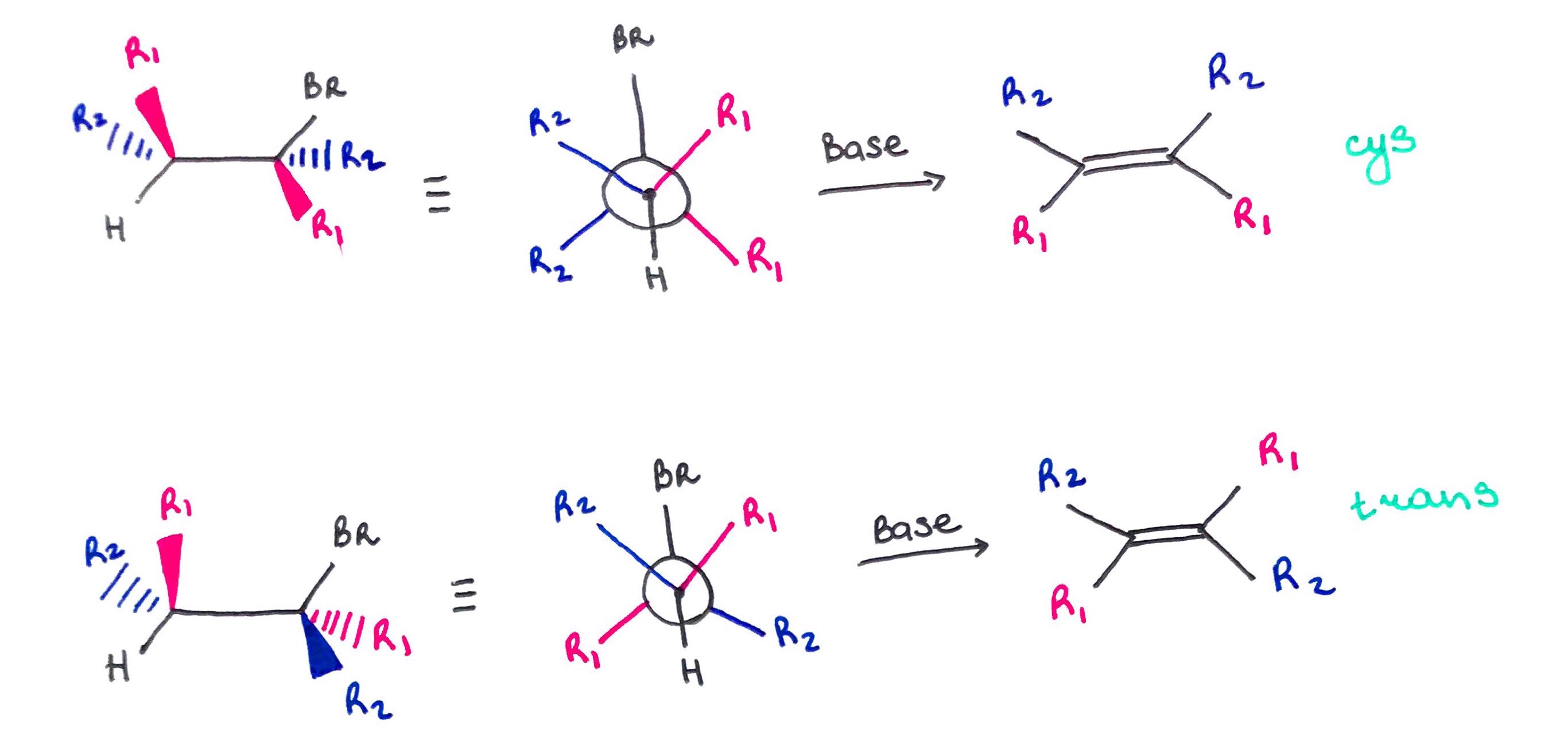
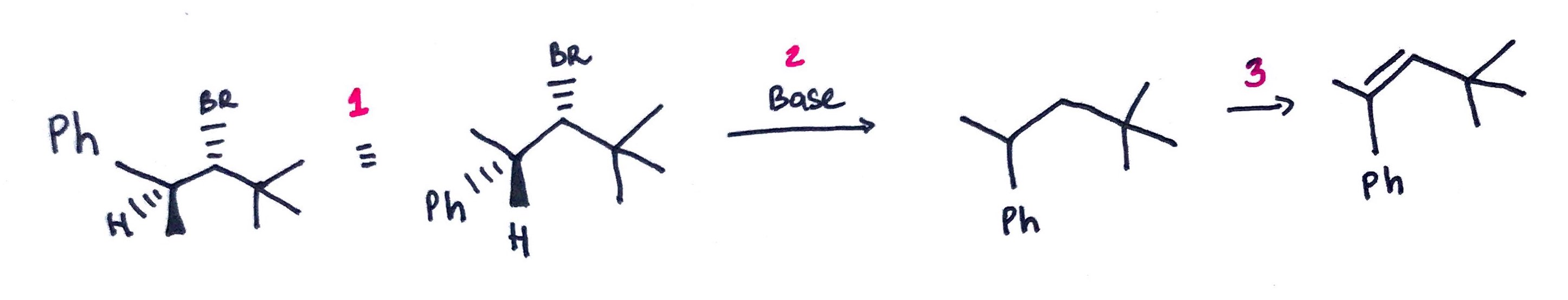
Quick and Dirty Method:
1. Rotate 3 atoms on one of the carbons to place proton or halide into anti-periplanar position.
2. Redraw the carbon skeleton without halide, proton and geometry. 3. Draw a double bond.
When LG in occupies axial position it is in anti-periplanar conformation with neighboring proton and the reaction can proceed.
Example: