Hybridization of atomic orbitals
Orbital hybridisation (or hybridization) is the concept of mixing atomic orbitals into new hybrid orbitals (with different energies, shapes, etc., than the component atomic orbitals) suitable for the pairing of electrons to form chemical bonds in valence bond theory. Hybrid orbitals are very useful in the explanation of molecular geometry and atomic bonding properties and are symmetrically disposed in space.
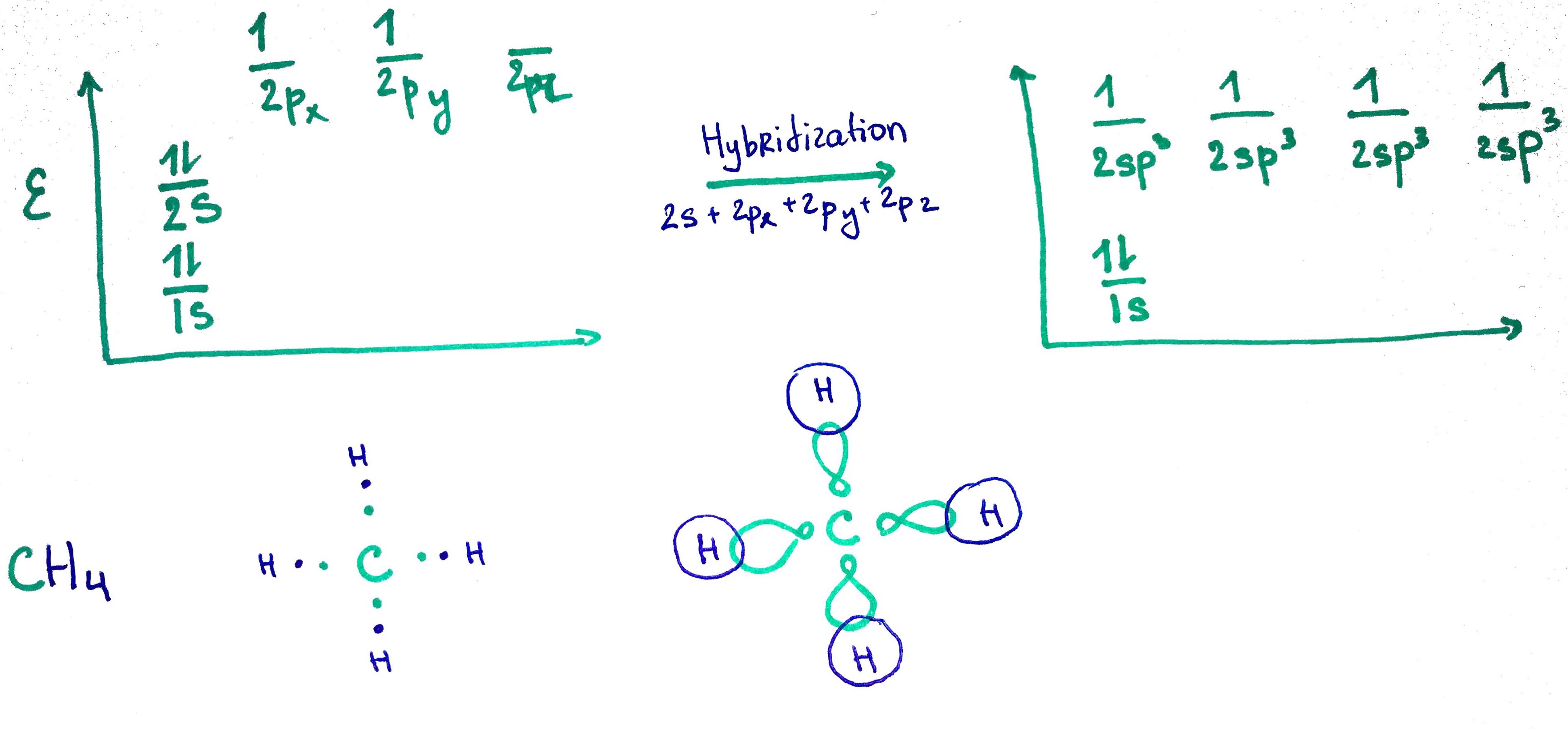
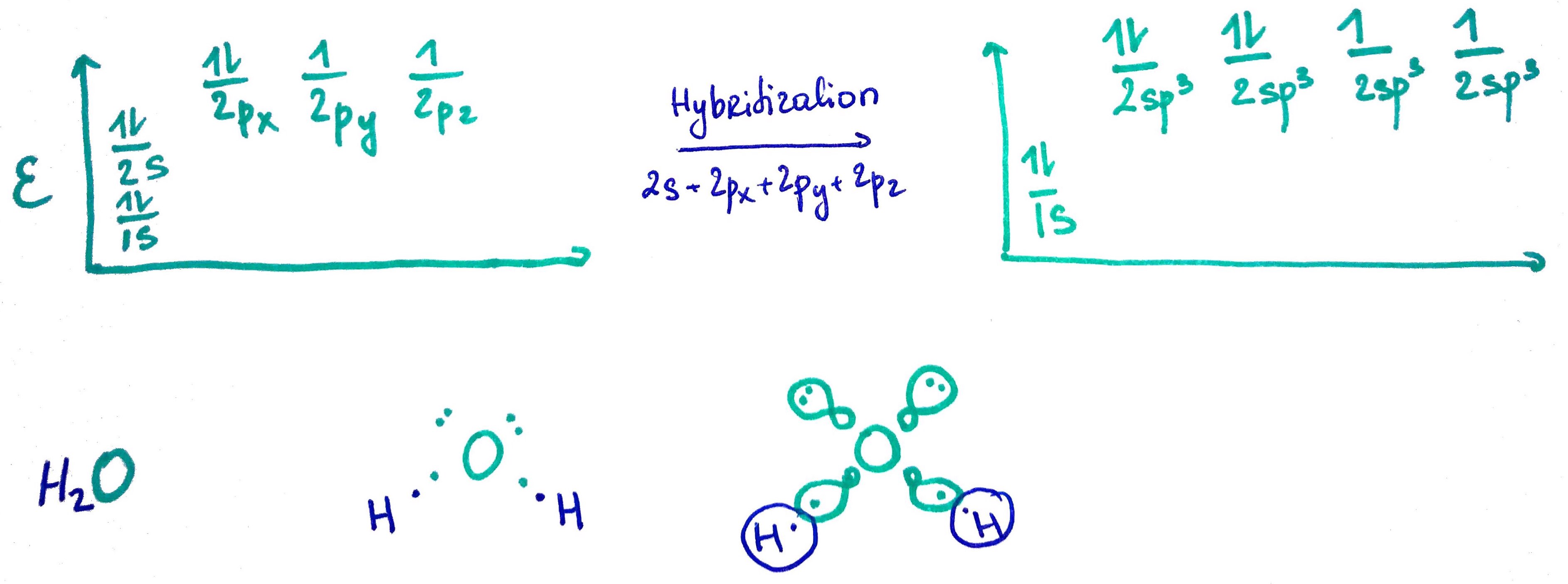
1. sp3 orbital
sp3 = s + p + p + p25% s
75% p
sigma bond σ - free rotation
σ bond lenght ~ 1.54A
Steric number = # of σ bonds + lone pairs
- Methane CH4 C electron configuration = 1s2 2s2 2p2
- Amonia N electron configuration = 1s2 2s2 2p3
- Water O electron configuration = 1s2 2s2 2p4
C steric number = 4
N steric number = 4
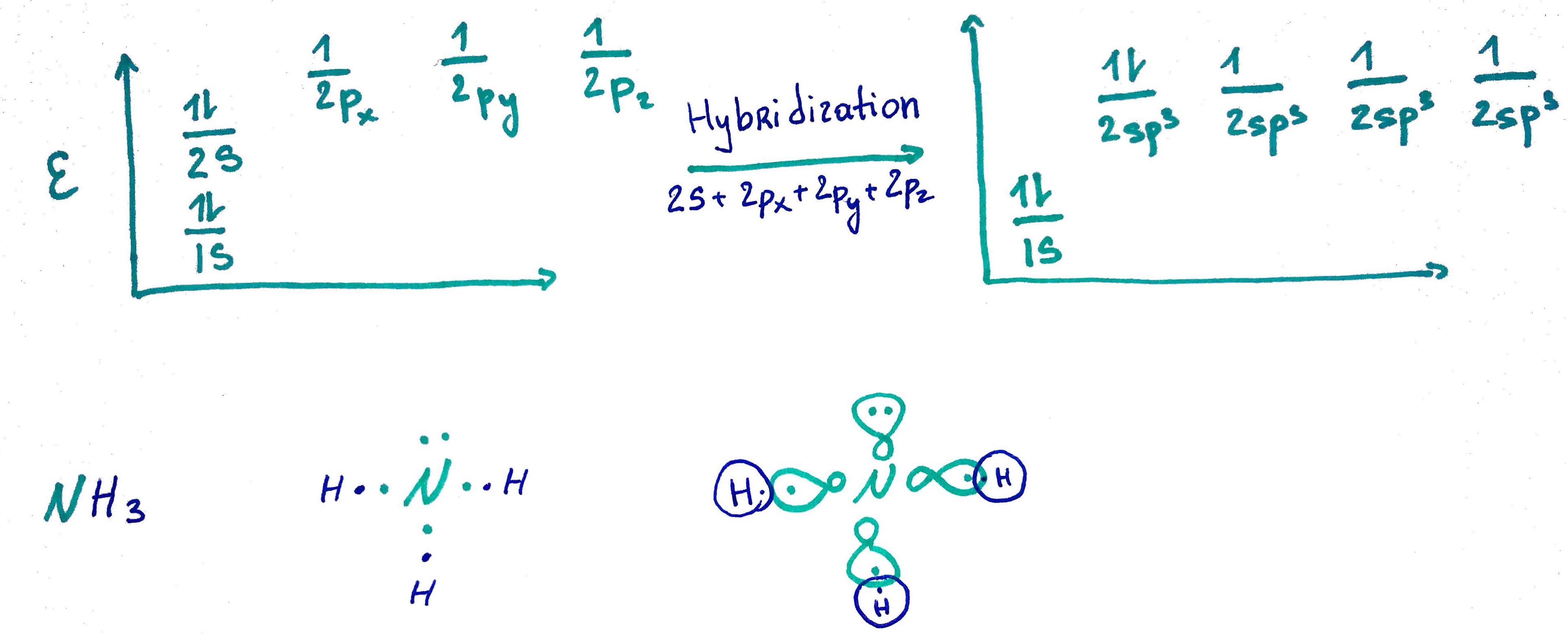
O steric number = 4
1. sp2 orbital
sp2 = s + p + p33% s
66% p
double bond = σ + π
no free rotation, planar
bond lenght = 1.34A
bond lenght decrease, because it has more s charachet and therfore is closer to nucleous
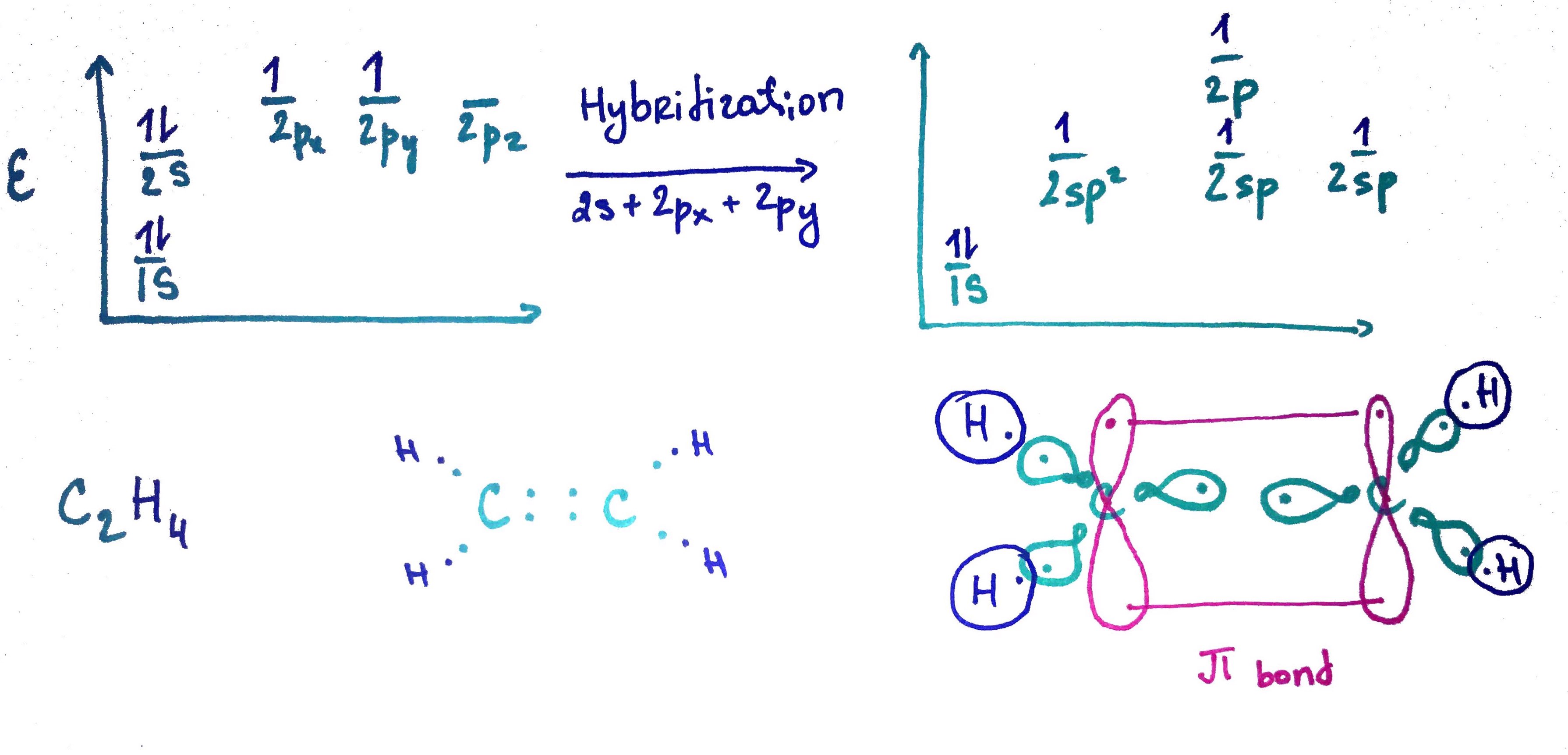
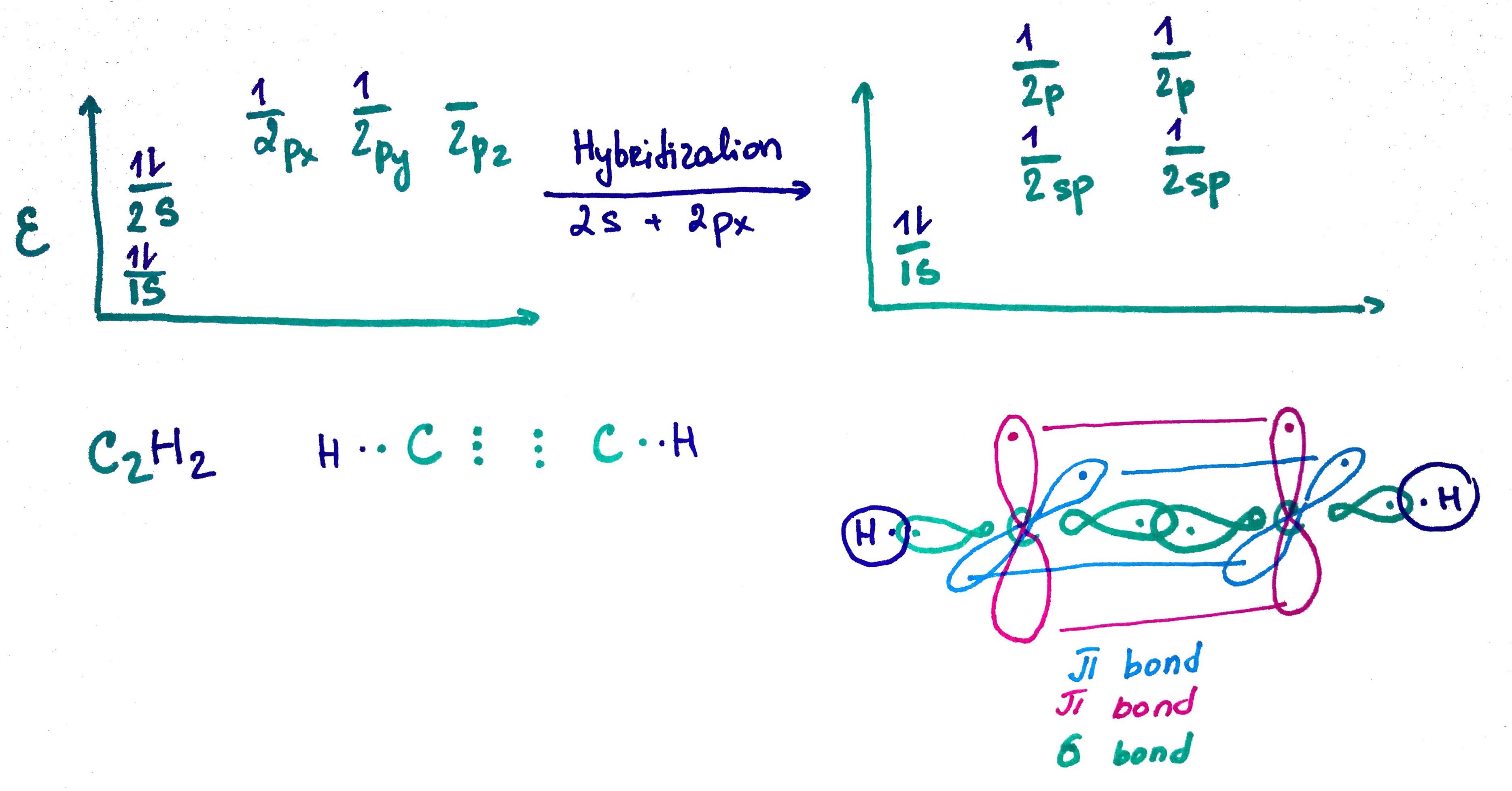
1. sp orbital
sp = s + p50% s
50% p
triple bond = σ + π + π
no free rotation, planar
bond lenght = 1.2A
bond lenght decrease, because it has more s charachet and therfore is closer to nucleous