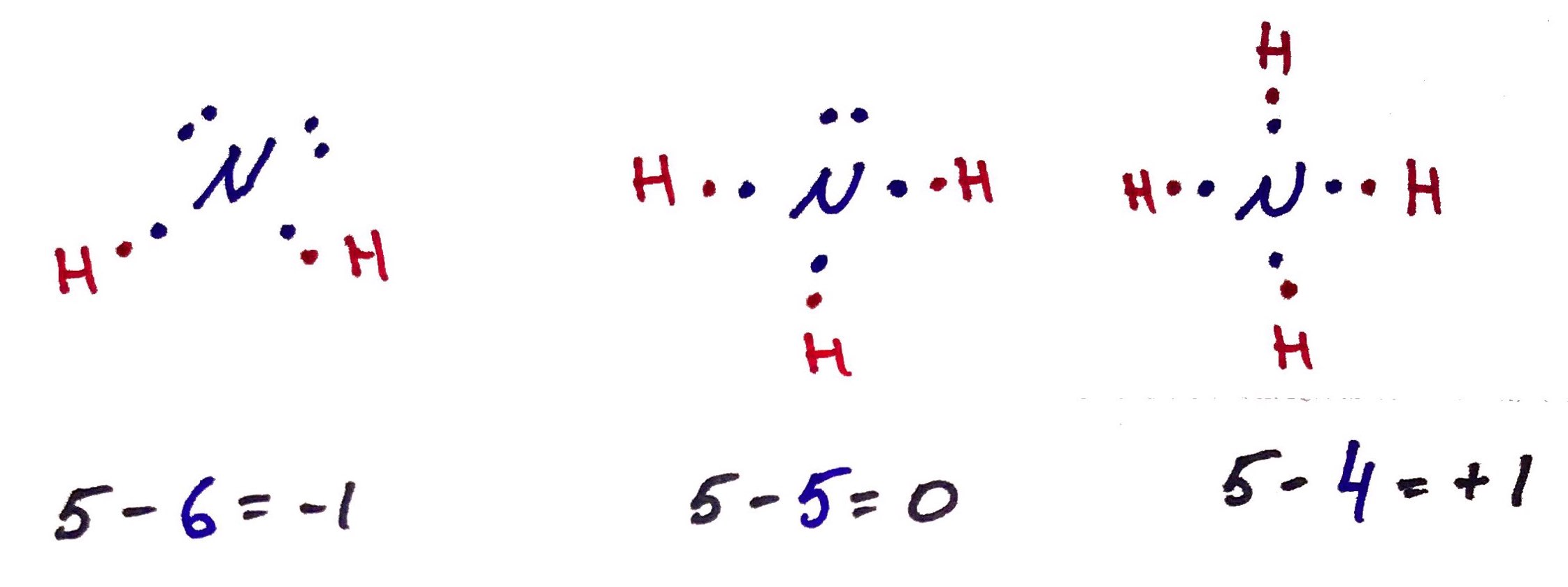Basic Bonding
1. Lewis Structers
- Total - # total valence electrons.
- Need - # of electrons needed to satisfy doublet/octet rule.
- Shared = Total - Needed (Number of electrons that participate in bonds)
# of lone pairs = Shared - Total; #/2
Practice
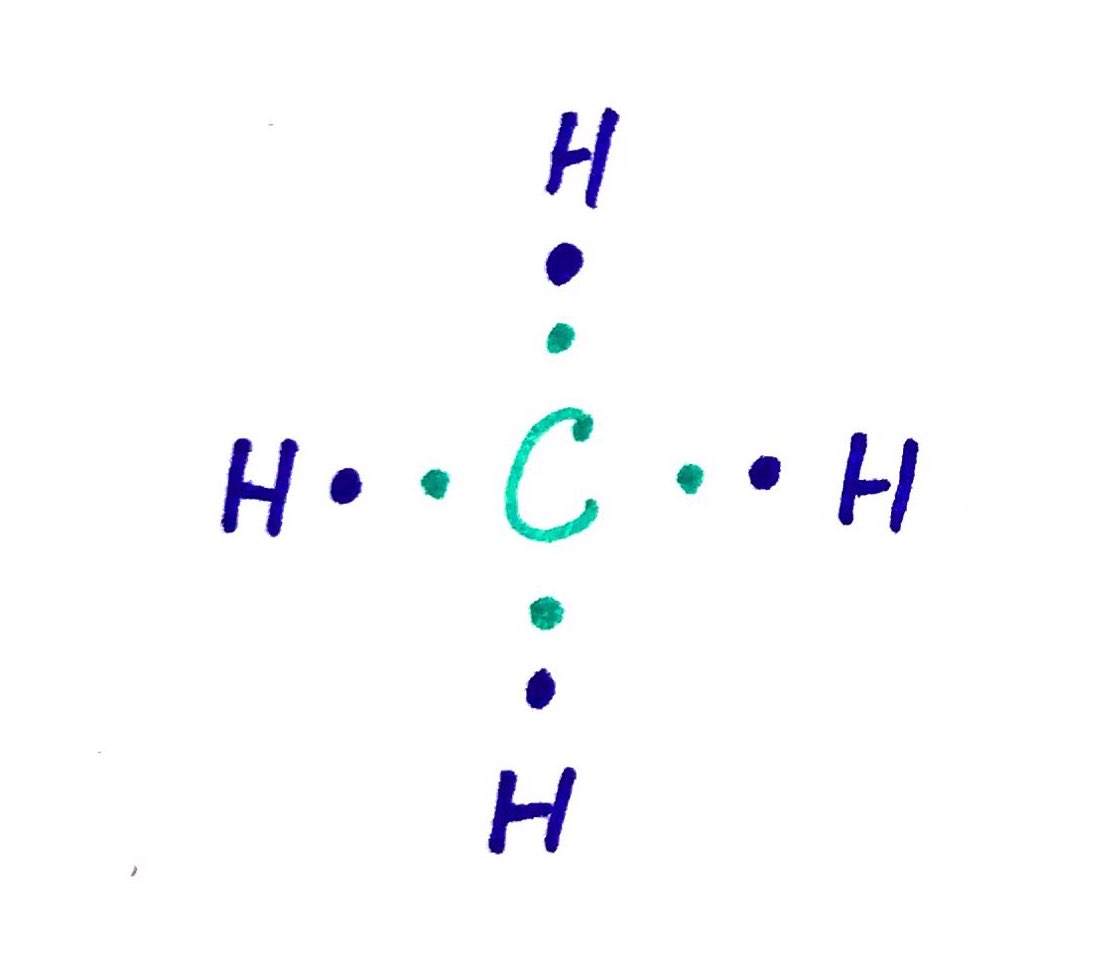
- Methane, CH4
- Total = C(1*4) + H(4*1) = 8
- Need = C(1*8) + H(4*2) = 16
- Shared = 16 - 8 = 8
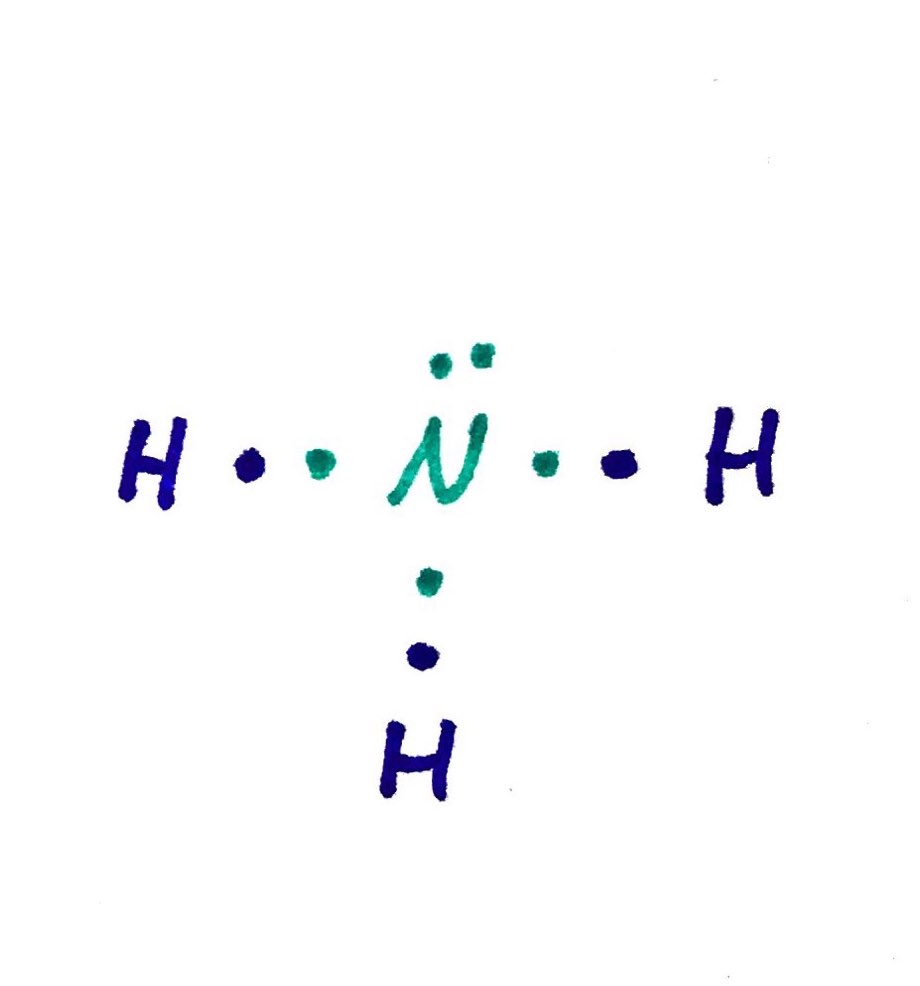
- Ammonia, NH3
- Total = N(1*5) + H(3*1) = 8
- Need = N(1*8) + H(3*2) = 14
- Shared = 14 - 8 = 6
# lone pairs = 8 - 8 = 0
# lone pairs = 8 - 6 = 2; 2/2 = 1
2. Formal Charge
Formal charge of an atom = (# valence electrons in ground state) - (# of valence electrons in bound state)
N atom in the ground state (as if found on the periodic table has 5 valence electrons.