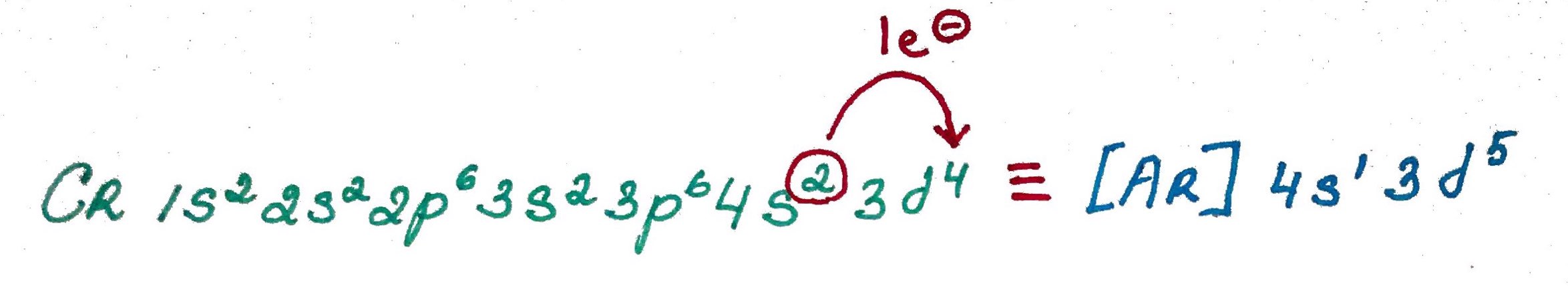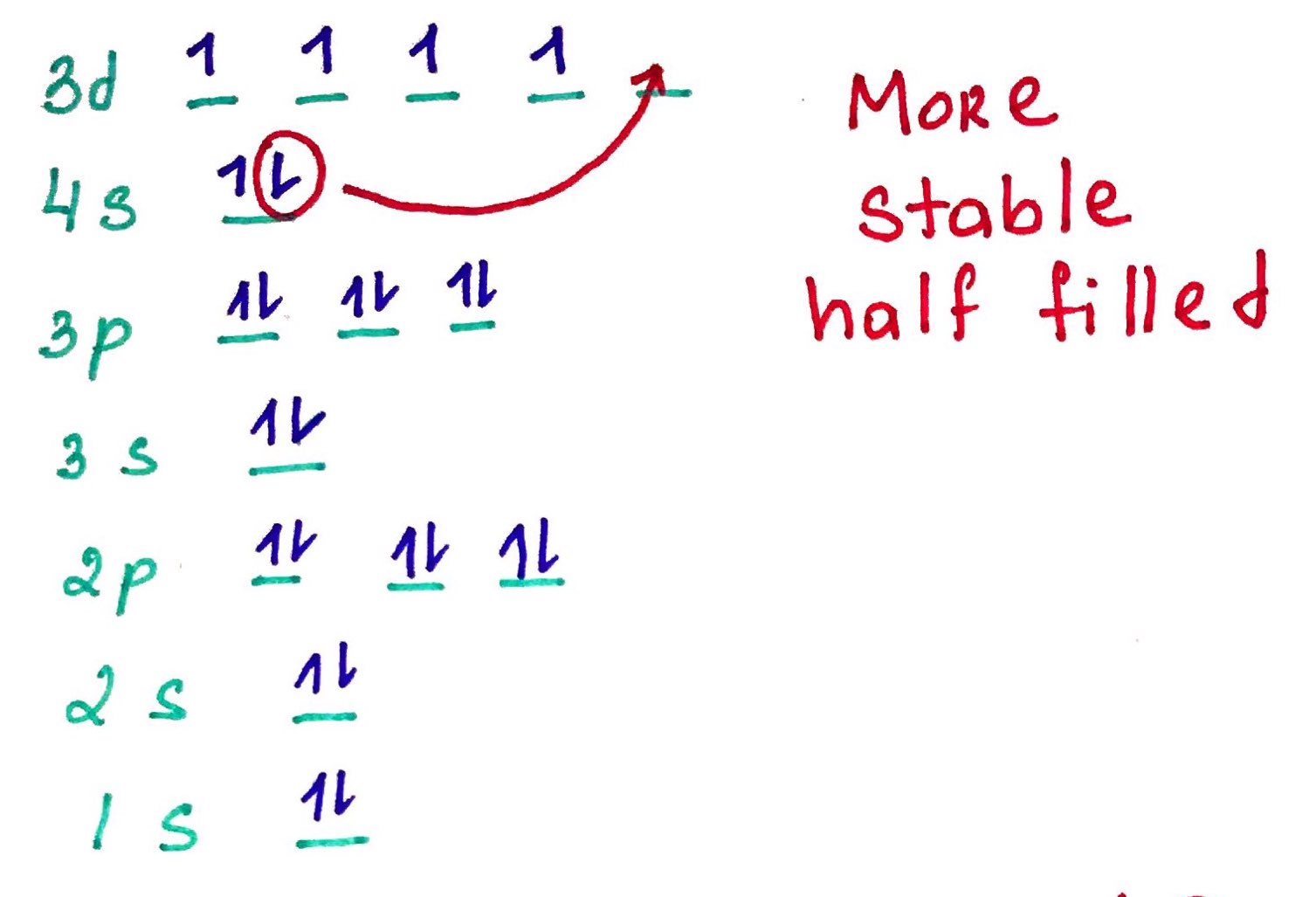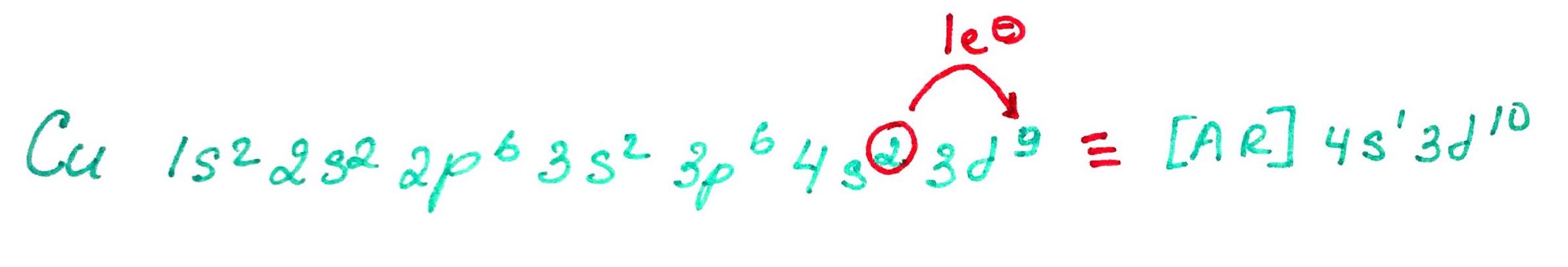Atoms and Orbitals
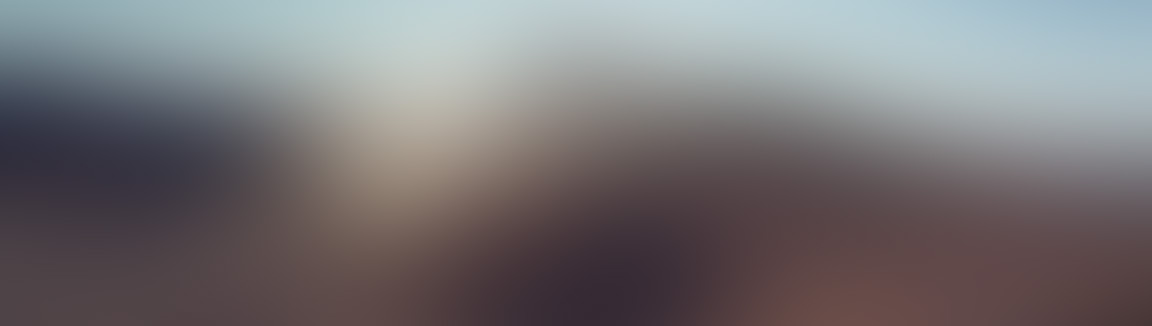
Overview
1. Subatomic particles.
2. Isotopes.
3. Quantum Numbers.
4. Electron configurations.
1. Subatomic particles.
An atom is a fundumential unit of matter. However it is not a smalest unit. An atom is composed of a proton and a neutron that constitute a nucleous and an electron that orbits around the nucleous.
Proton is positivley charged.
Neutron has no charge
Electron has negative charge
All elements on the periodic table can be defined by these 3 subatomic particles.
For convince chemists use following definitions:
Atomic number (Z) = number of Protons
Mass number (A) = number of Protons + number Neutrons
2. Isotopes.
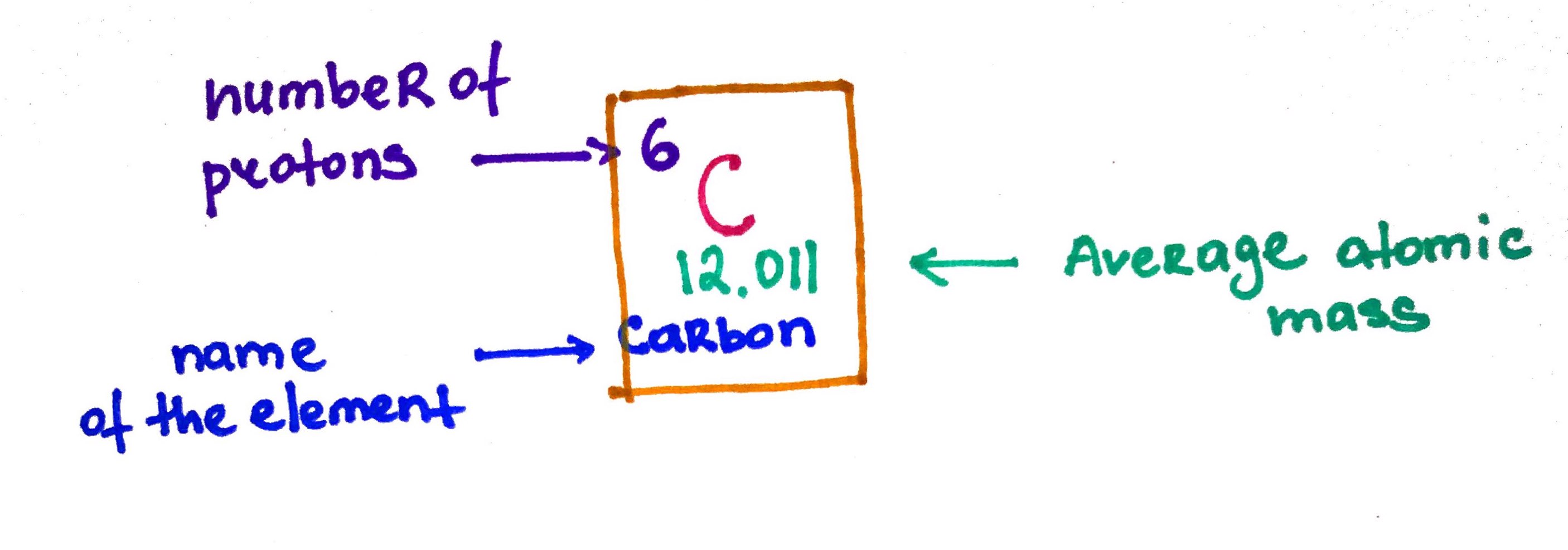
Isotopes are atoms with the same number of protons but different number of neutrons. For example carbon has two isotopes Carbon-12 and Carbon-13. If you take a sample of carbon it will always contain these two isotopes at different proportions. These proportions are known as fractional abundance.
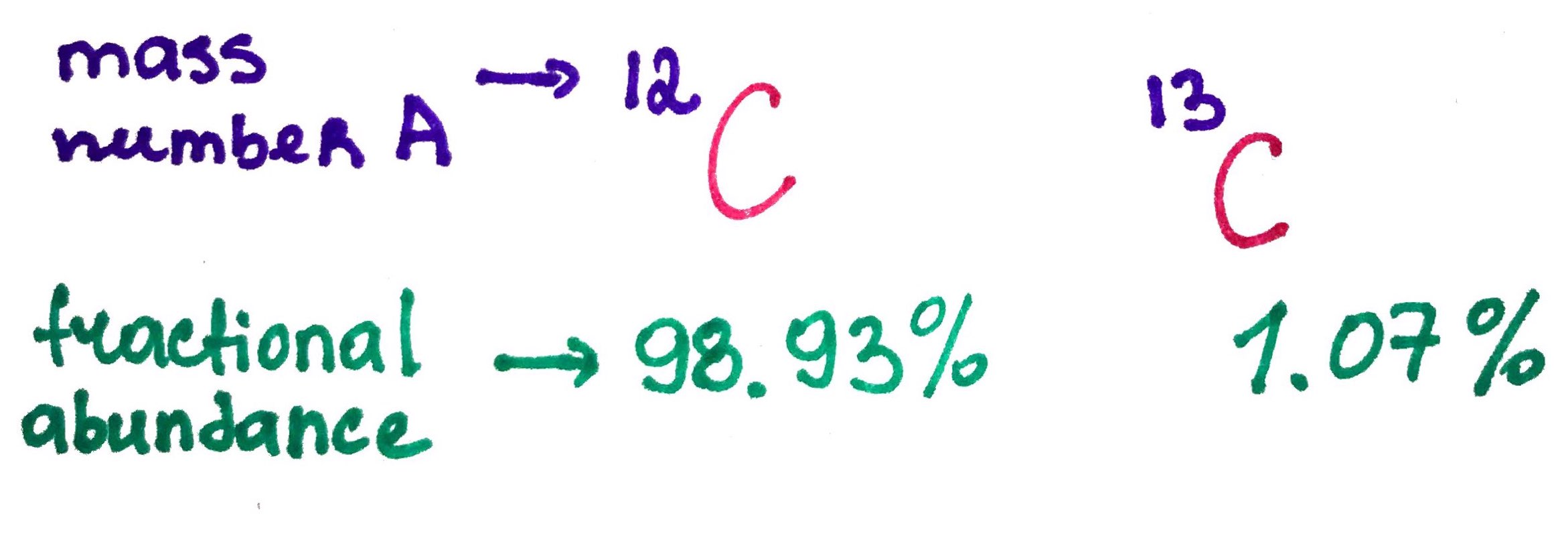
Often students asked to calculate the average atomic mass for an element.

How to calculate it?
Calculate average atomic mass for carbon that has follwoing isotopes:
Carbon-12, atomic mass = 12amu, fractional abundance = 98.93%
Carbon-13, atomic mass = 13.003amu, fractional abundance = 1.07%
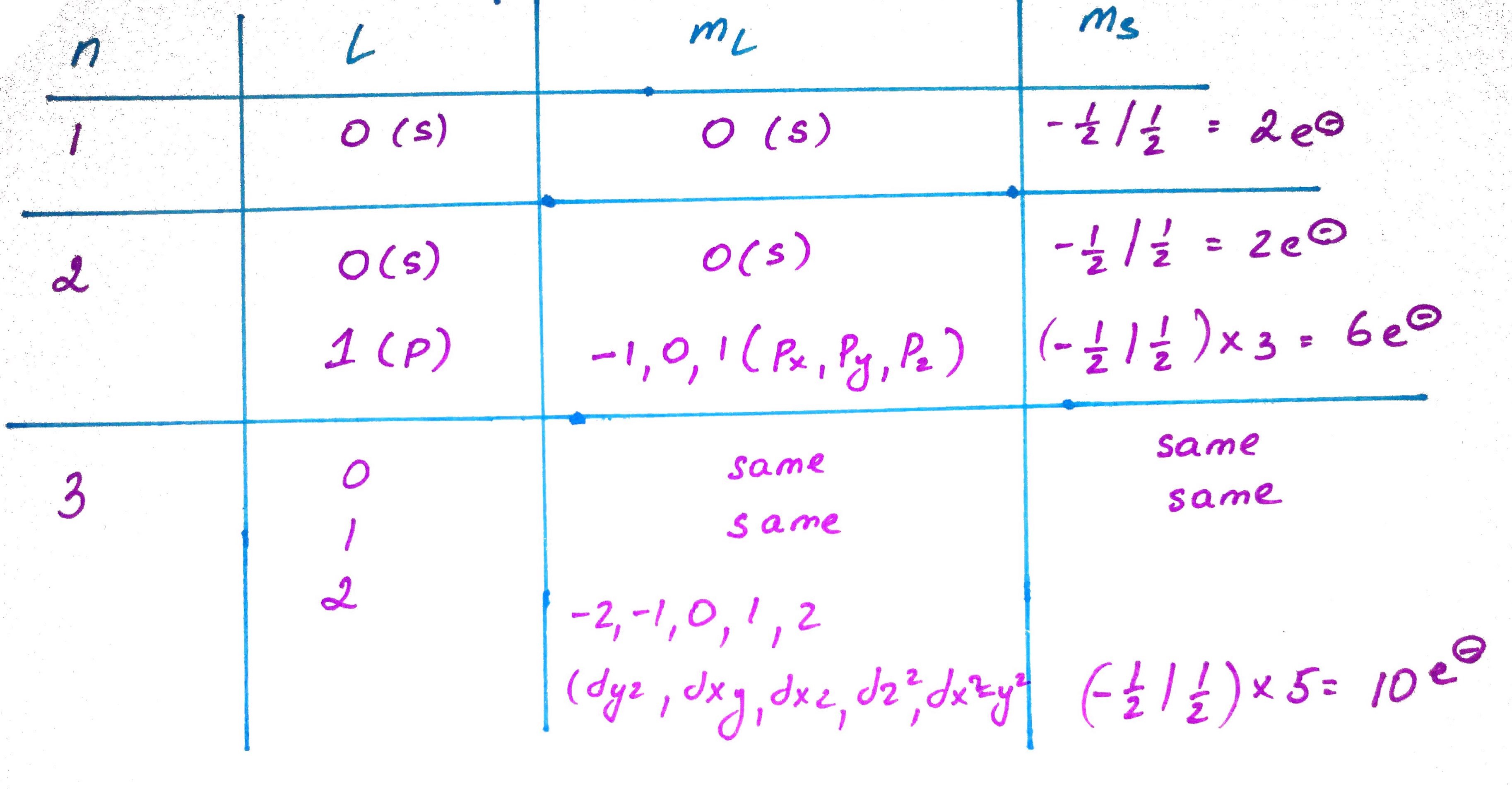
3. Quantum Numbers.
- N - principle number, shell
- L - angular momentum number, subshell
- Ml - magnetic quantum number
- Ms - electron spin number
Main energy shell number, describes how far away the electron is from the nucleous.
Describes the shape of the orbital (s, p, d, f)
Describes the orienatation of the orbital (px , py , pz)
Describes the electron "spin" (up or down)
Practice
Identify the subshell in which electrons with the following quantum numbers are found:- n=2, l=1
- n=4, l=2
- n=6, l=0
First determine the subshell/orbital shape (l)
Second determine the shell (n)
Answears:
- 2p
- 4d
- 6s
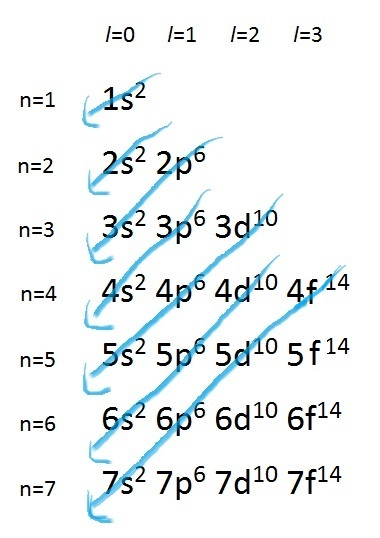
4. Electron configurations.
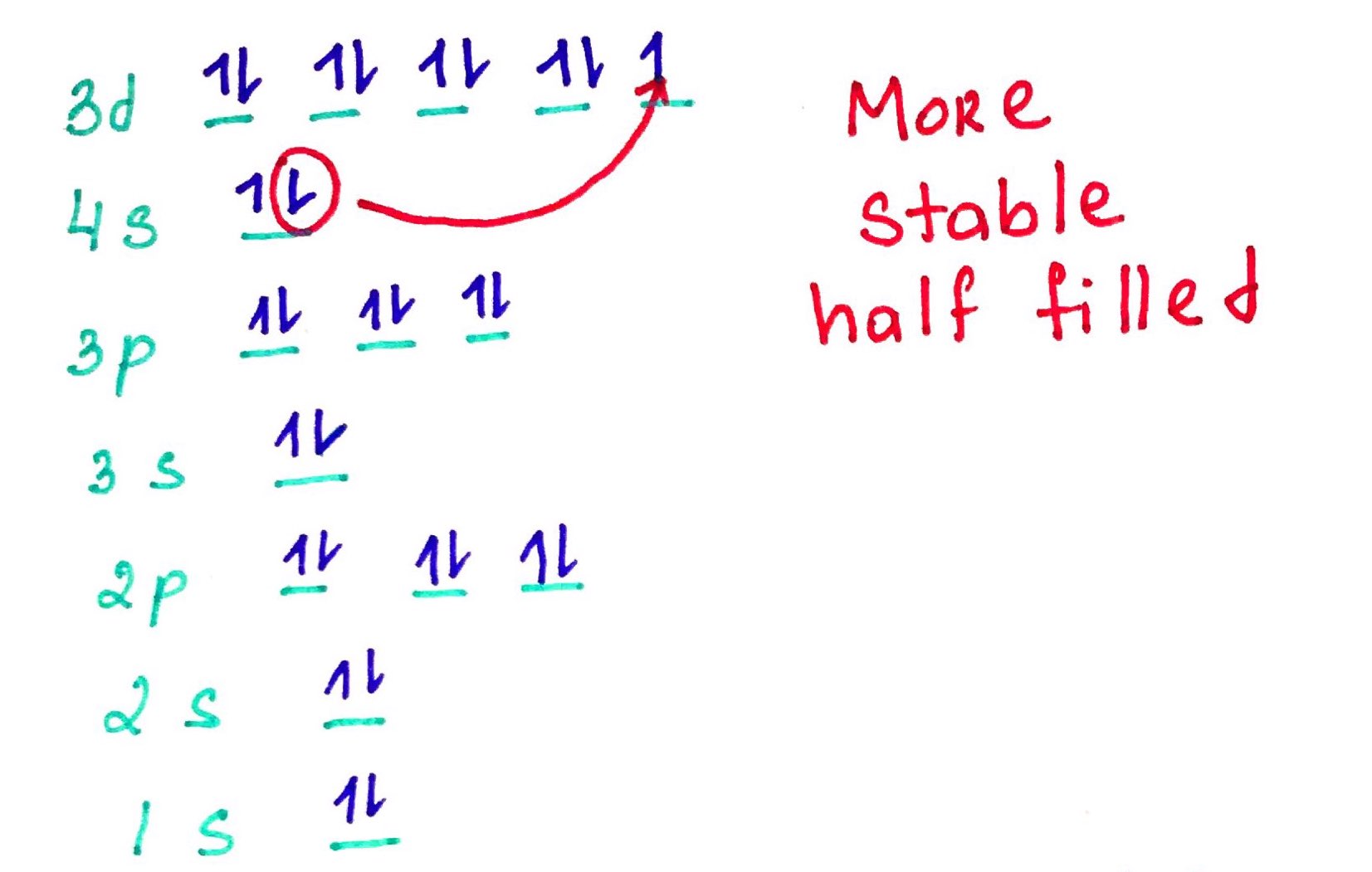
Electron configurations rules:- Aufbau principle - German Aufbauprinzip (building-up) states that electron will occupy the lowest energy orbital.
- Pauli exclusion principle - no 2 electrons in the same atom may have identical set of qunatum numbers (meaning if 2 electrons on the same orbital one of them must be up and the other down.
- Hands rule - every orbital in a subshell is singly occupied with one electron before any one orbital is doubly occupied, and all electrons in singly occupied orbitals have the same spin (all up or all down).
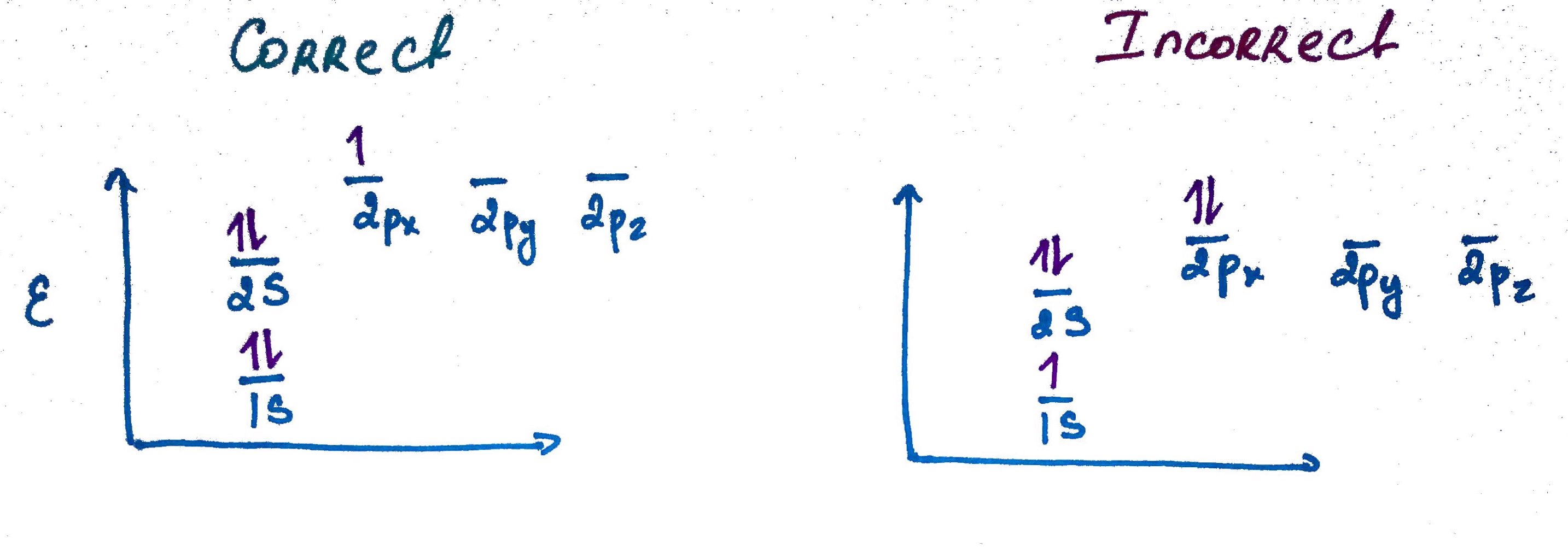
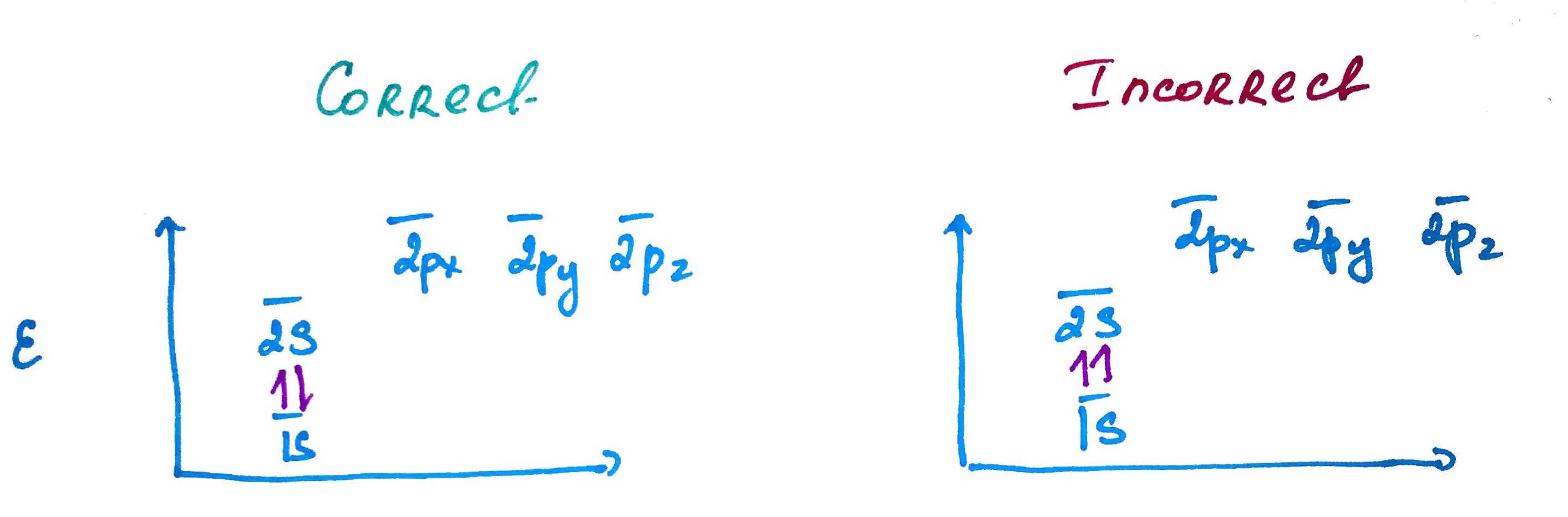
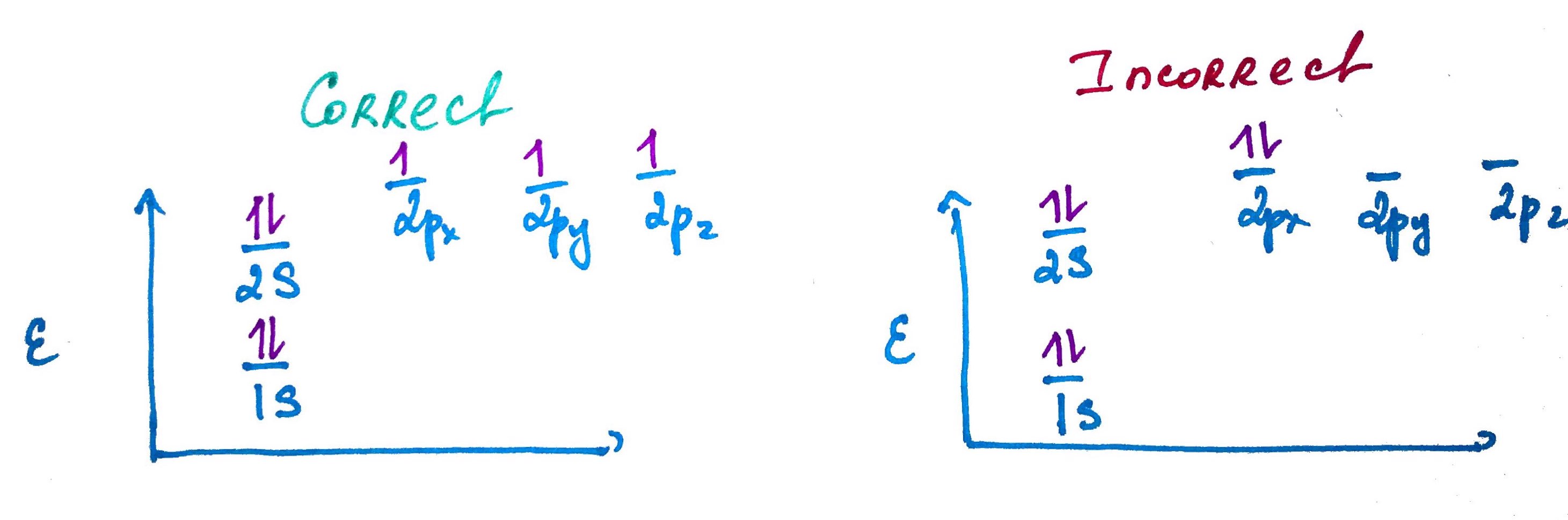
Quick Trick of electron configuration digram bulding: