VSEPR
1. VSEPR
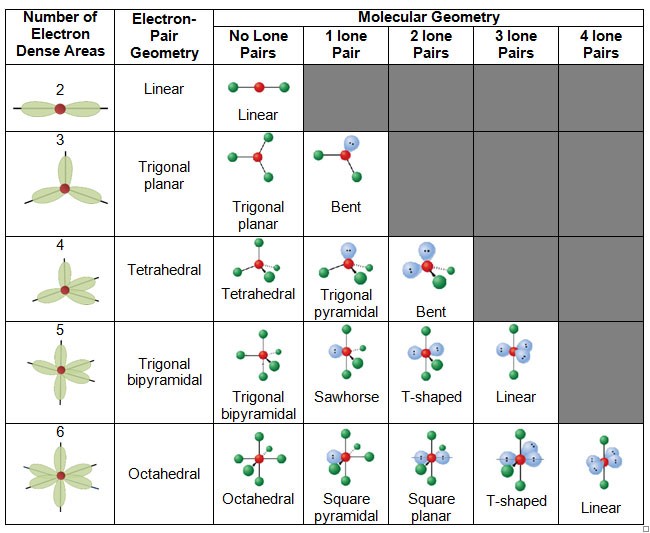
VSEPR - Valence shell electron pair repulsion theory.
Is a model used to predict the geometry of individual molecules from the number of electron pairs surrounding their central atoms
- Draw a lewis structure.
- Count the nuber of electron clouds, surounding the central atom.
- Predict the geometry with electron pairs.
- Ignore lone pair and predict the molecular geometry
Practice
1. CO2 - Linear
Valence = 4 + 6*2 = 16
Needed = 8*3 = 24
Shared = 24 - 16 = 8
Bonds = 8/2 = 4
Lona pairs = 16 - 8 = 8; 8/2 = 4
2. BH3 - Trigonal Planar
3. BH2(-) - Bent
4. CH4 - Tetrahedral
5. NH3 - rigonal Pyramidal
6. H2O - bent
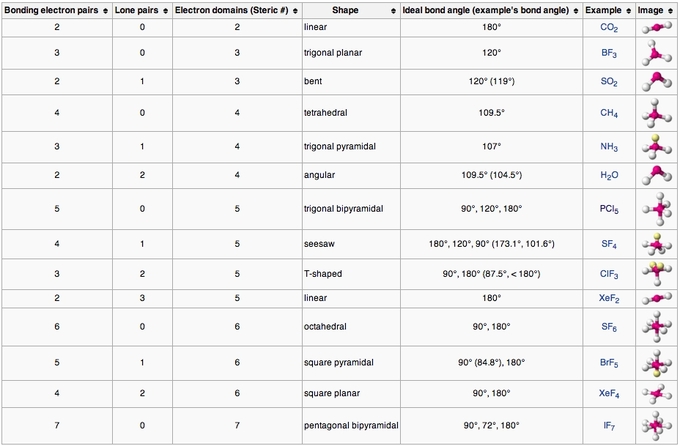
2. VSEPR Bond Angels
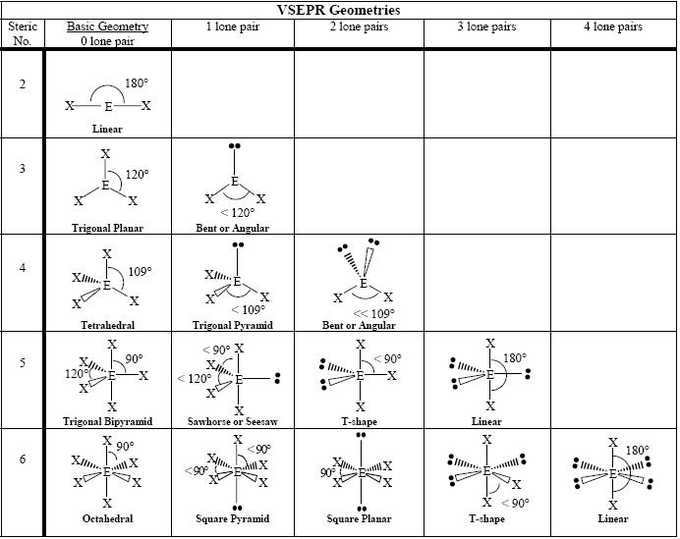
2. VSEPR Examples