Fundamentals Of Biochemistry
1.Properties of life.
Living organisms are:- Complex and highly organized.
- Composed of functional structures. (Mitochondria function as a power house of the cell, hands are meant to grab).
- Actively engaged in energy transformations. (Potential energy stored in food is transformed into kinetic energy during running)
- Capable of self-replication and regeneration.
- Composed of cells.
![]() by pngkey.com
by pngkey.com
Emergence:
Is a property of living organisms that states that a whole organism have properties that its individual parts do not have. I find it intuitive to think of emergence in terms of pixel art. Individually pixels are just colored squares, however when arranged in a certain way they create a picture that posses new properties, like shape and cuteness. Likewise proteins and DNA create cells. Cells posses properties that individually DNA and proteins do not have, such as cell motility and cell division.
Vitalism
Was the main belief among scientist 18th/19th centuries, that was meant to explain the difference between living and non living. Central idea of vitalism declares that living organisms contain some sort of "vital spark" or "elan vital" as it was originally defined by French philosopher Henri Bergson, and therefore living organisms are fundamentally different from non living matter in that sense.
Wöhler UreaVitalism was challenged by the the synthesis of Urea by Friedrich Wöhler. Urea is molecule that living organism use to excrete nitrogenous waste. If you were a scientist in the 19th century it would be logical for you to assume that Urea could only be found in the living organisms, because only they posses the "vital spark" that is needed for the synthesis of this molecule. Wöhler accidentally was able to synthesize Urea in a laboratory in a tube outside of a living organism. The result of this experiment challenged the then prevalent idea of vitalism.
Buchner-Manaseina yeast extract fermentationAnother experiment delivered a mortal blow to vitalism. For centuries people were making alcohol by yeast fermentation. According to vitalism, the process of fermentation is possible because living yeast posses the "vital spark". In other words if you kill the yeast you would kill the "vital spark" and fermentation should stop. Eduard Buchner and Maria Manaseina showed that killed yeast are still able to catalyze alcohol fermentation.
Miller-Urey experimentArguably, one of the most known experiments in biochemistry. Experiment demonstrated that many of the molecules necessary for the emergence of life could be formed from the simple molecular precursors (water, ammonia, hydrogen and methane) that were thought to be present on the early earth. The gasses were mixed and exposed to an electrical spark – simulating lightening. More than 20 amino acids and other biological molecules were found in the resulting mixture.(N. Astorf)
Conclusion: Together these three experiments challenge the theory of vitalism.
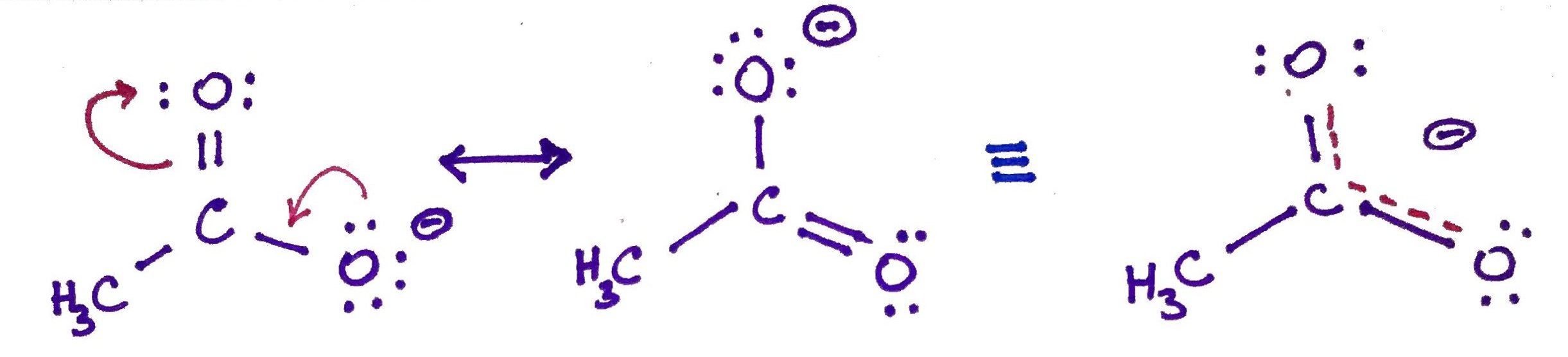
2.Resonance.
3.Tautomers.
Carbonyl compound with a Hydrogen in alpha position will be in the equilibrium with it's own enol (from alkene and alcohol). This reaction called Keto-Enol Tautomerization can be catalyzed either by Acid or Base.
Beware: Unlike a molecule in resonance, tautomers are different molecules that exist in equilibrium with each other.Beware: Usually Ketone form is more stable, because the carbon-oxygen double bond is stronger than the carbon-carbon double bond.
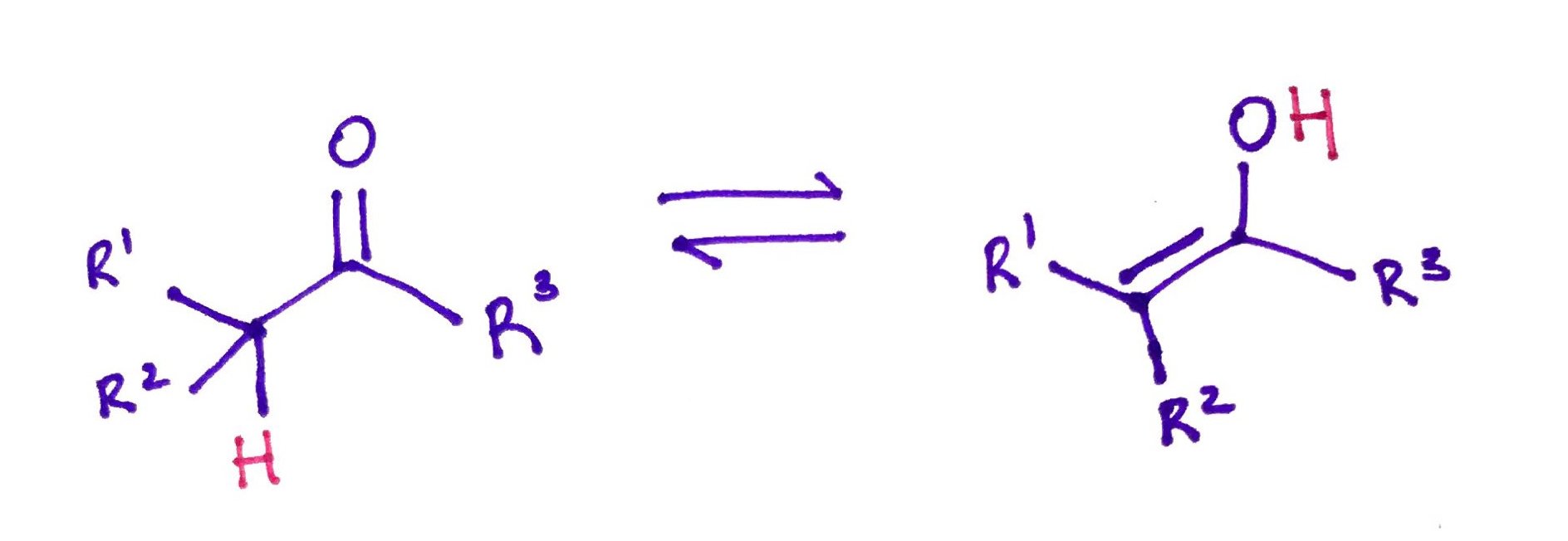
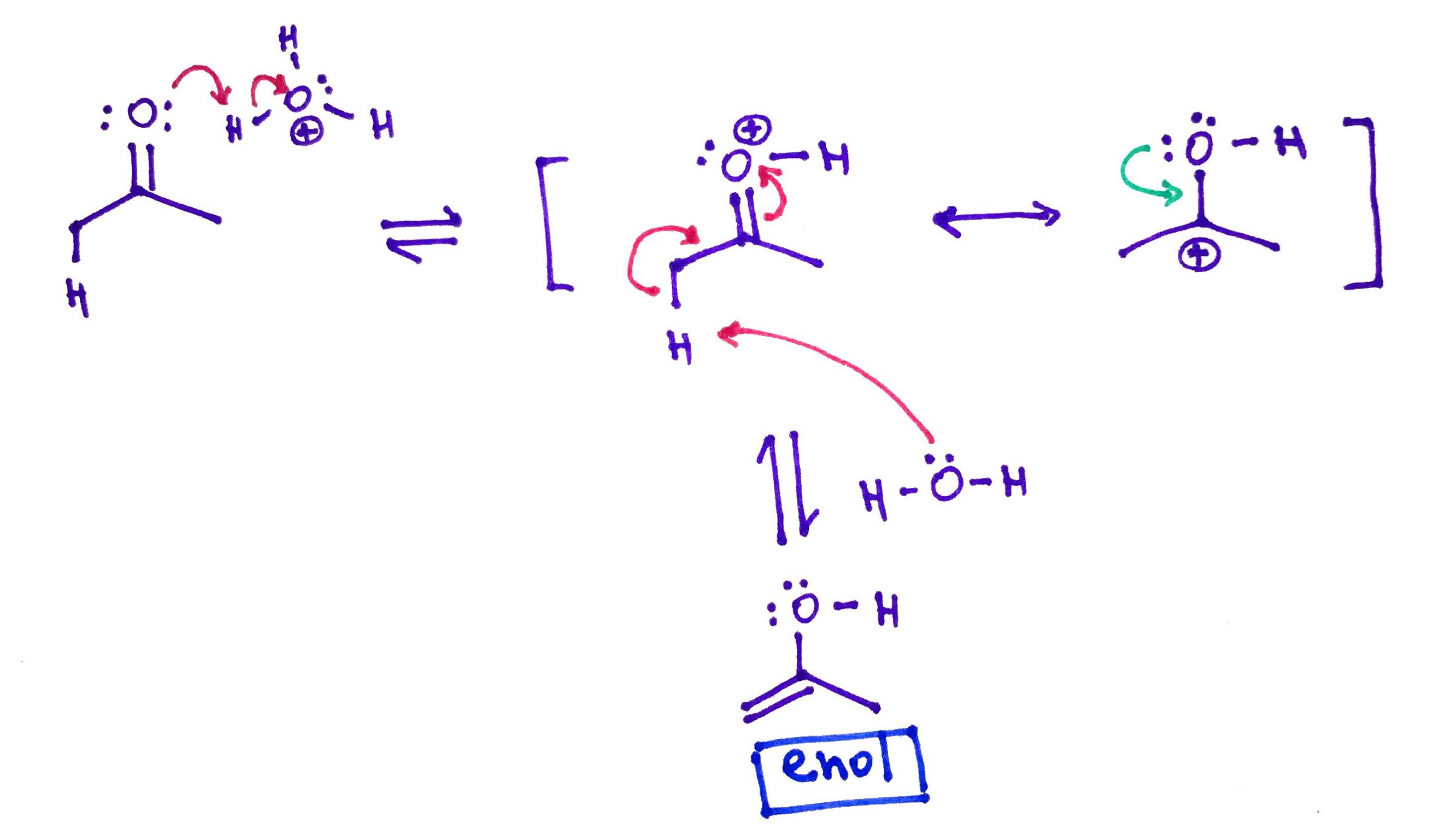
Acid catalyzed.
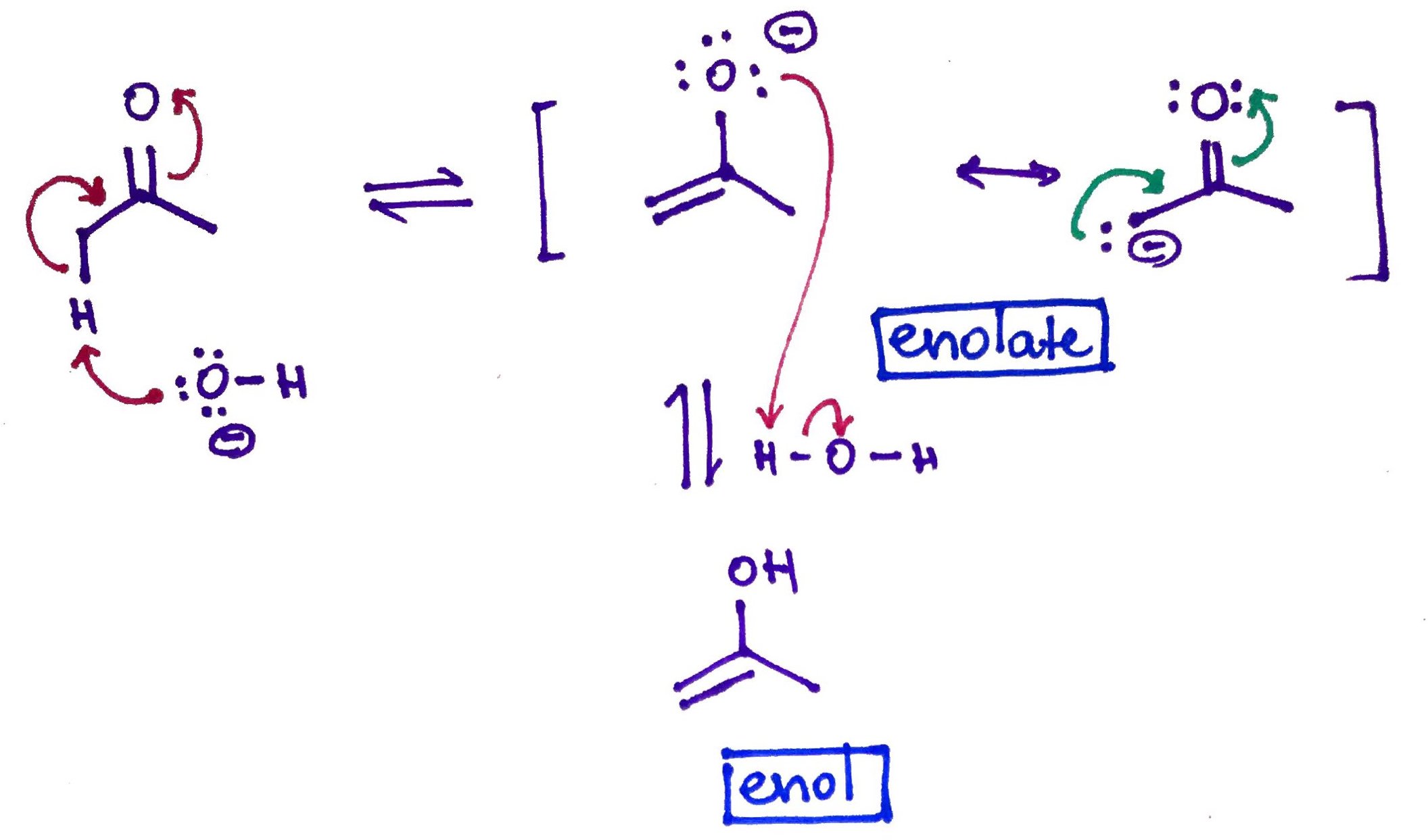
Base Catalyzed. Notice enolate ion formation.
4.Aldol Condensation
When enol or enolate reacts with a carbonyl compound β-hydroxyaldehyde or β-hydroxyketone is formed in a reaction called Aldol Condensation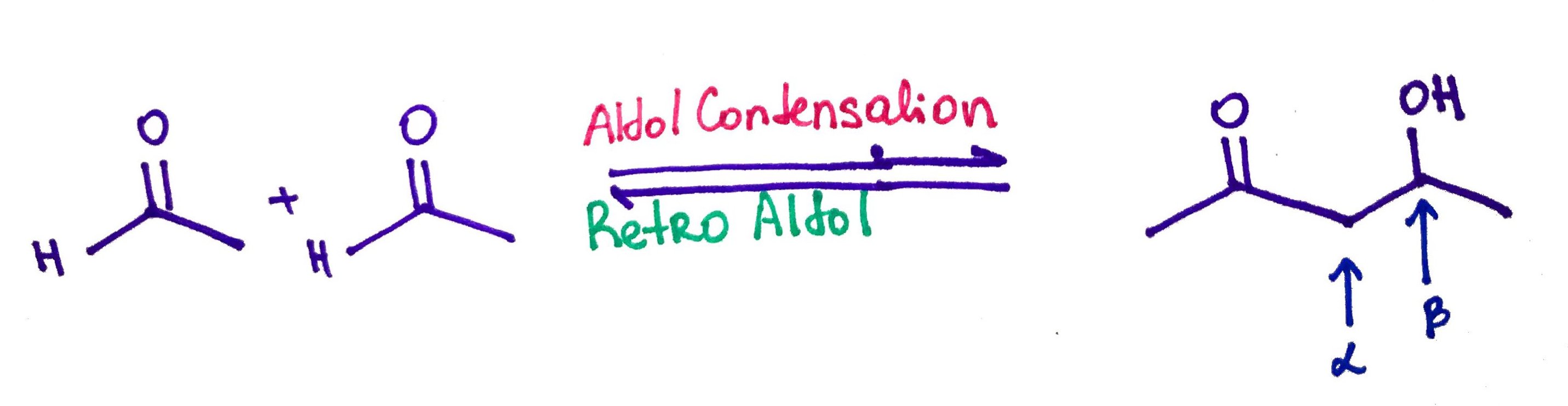
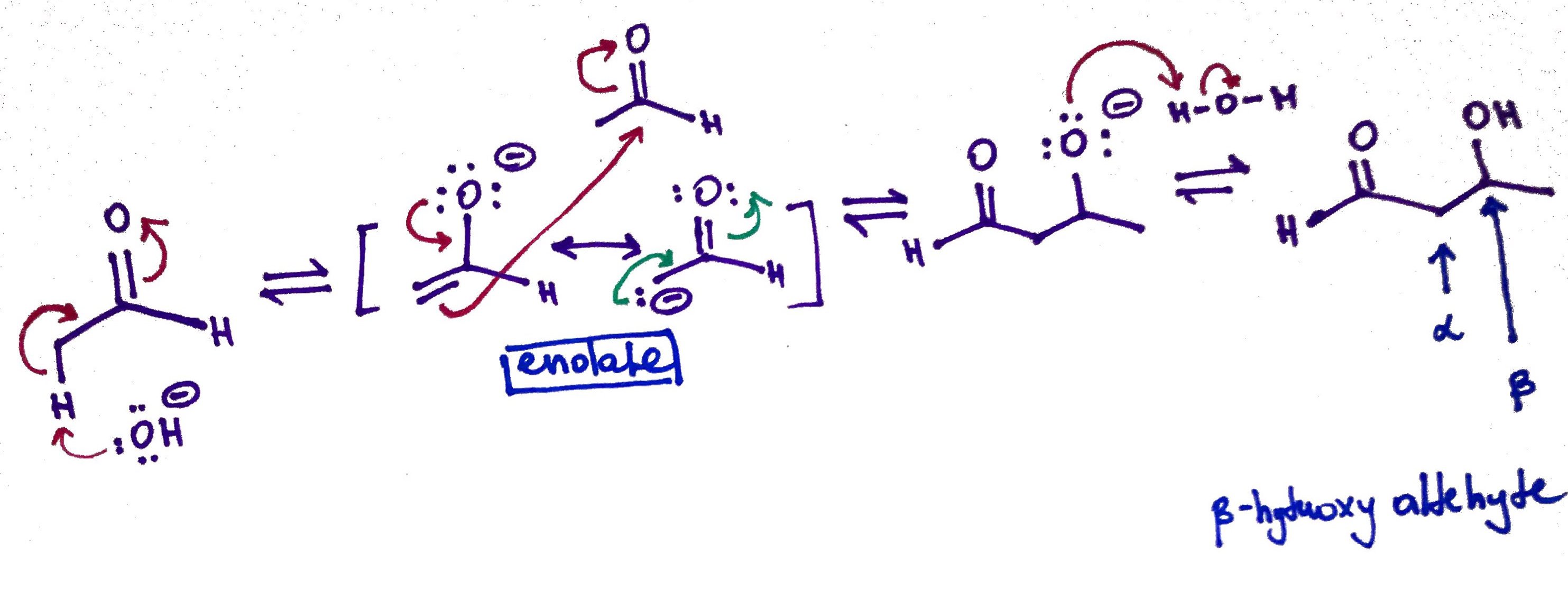
Aldol Condensation.
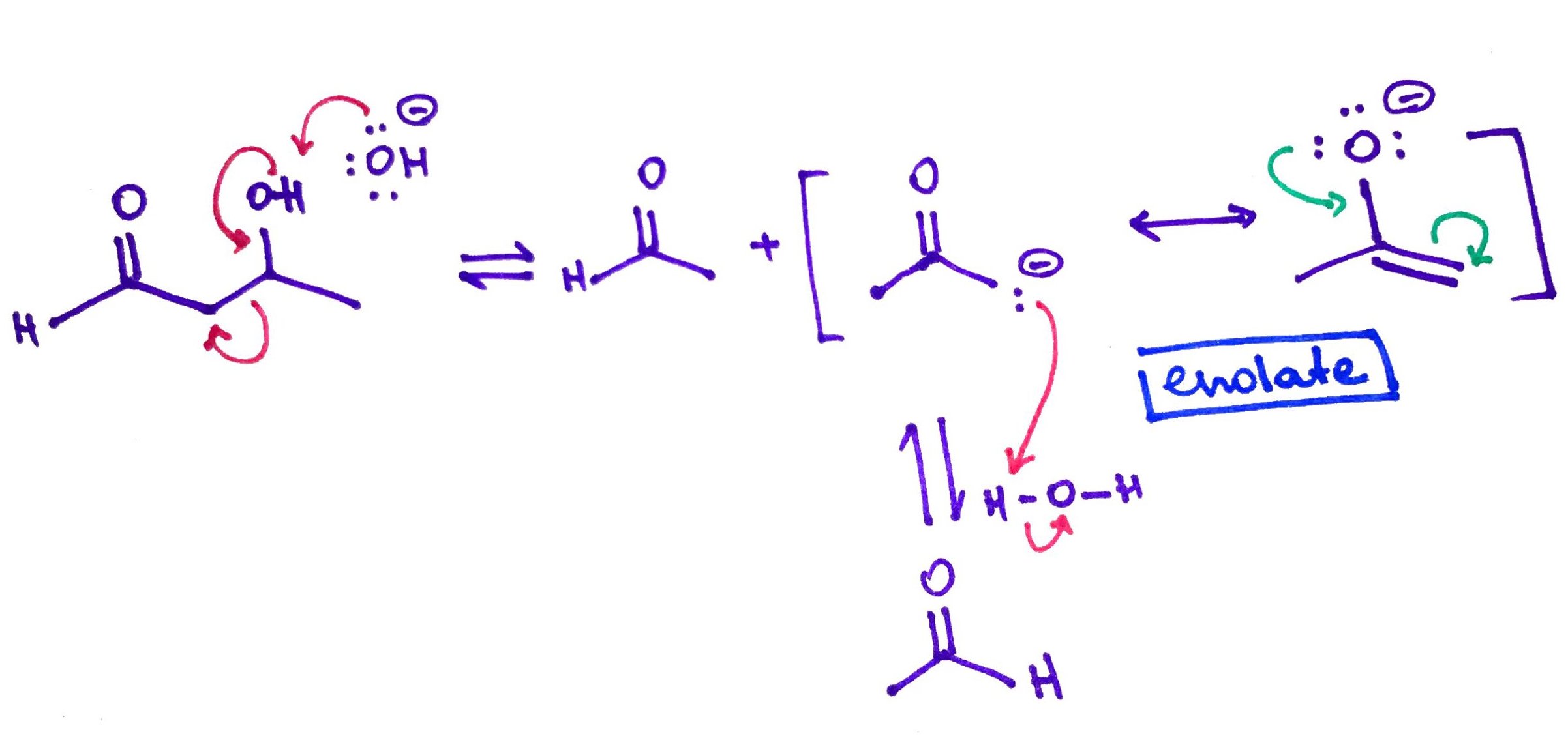
Retro Aldol.
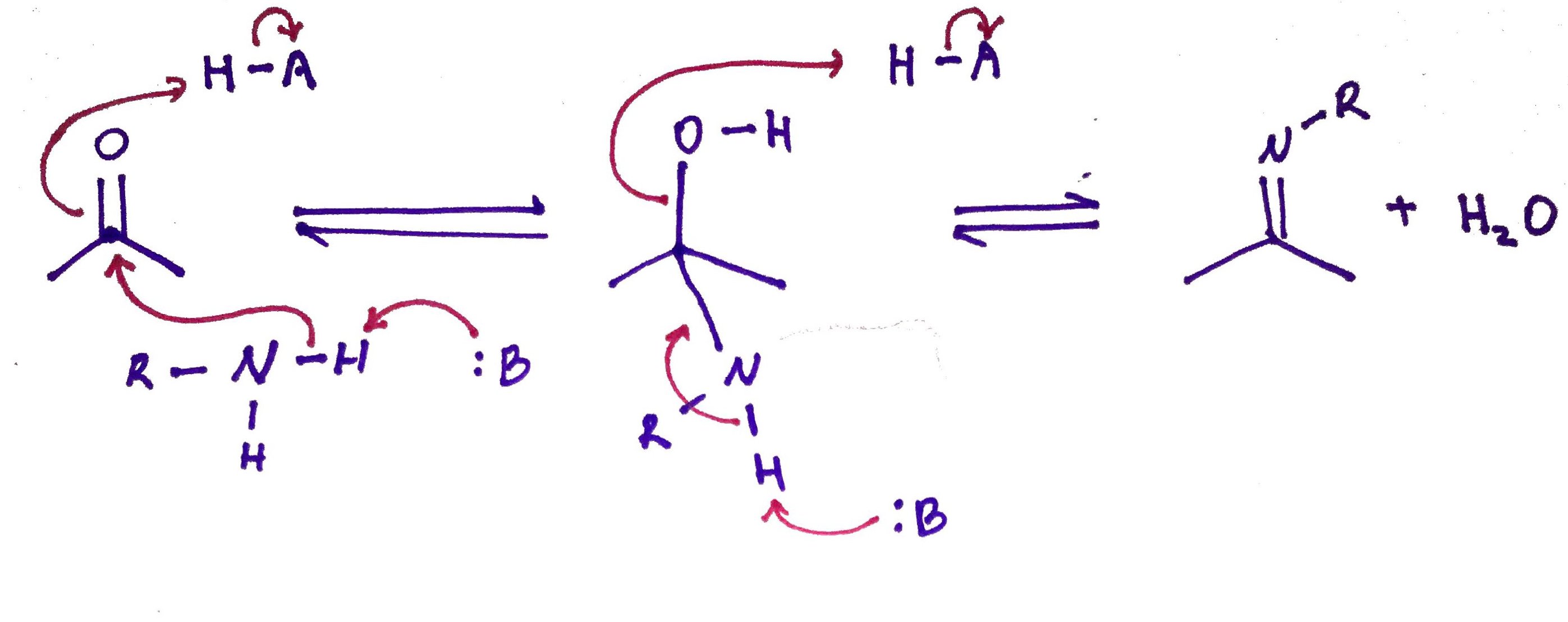
5.Schiff Base.
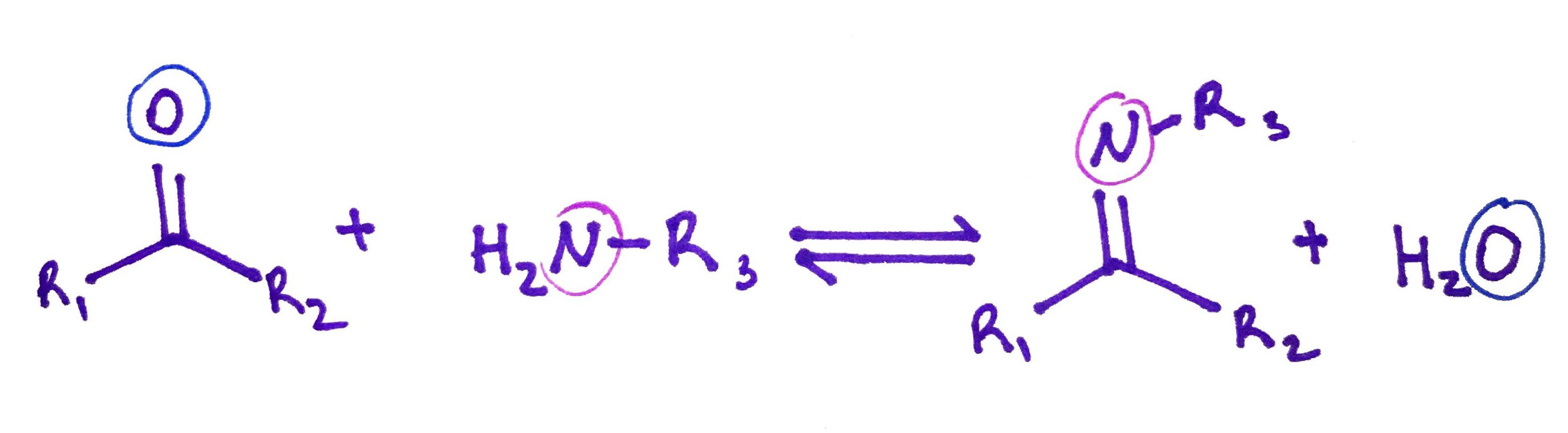
Schiff bases are imines in which R3 is not hydrogen. R1 and R2 could be hydrogens.
6.Oxidation Numbers.
Oxidation number conceptually represents the amount of energy in the molecule with 8 being the highest and 0 the lowest.
When we combust methane it becomes oxidized and the energy is released
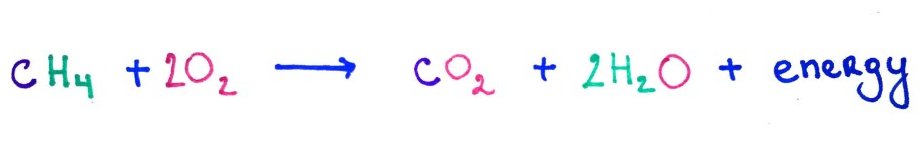
We can assign oxidation numbers to atoms, by assuming that all the bonds in a molecule are ionic, meaning they do not share electrons like covalent bonds do. Then the atoms will get electrons based on their electro negativity. Carbon is more electronegative than hydrogen, so it will keep the electrons. Follow the example below.
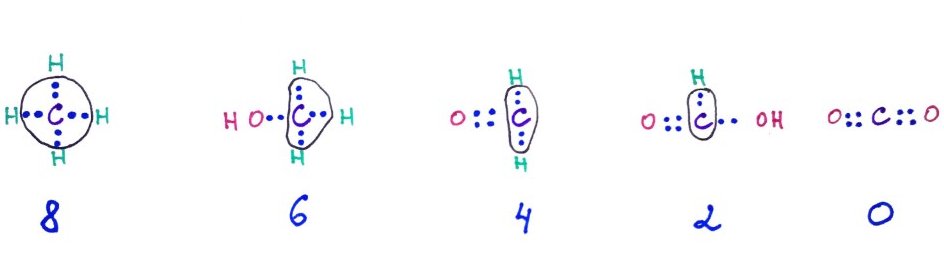
From left to right: Oxidation numbers for carbon atom in Methane, Ethanol, Formaldehyde, Formic acid, Carbon dioxide. The electrons that belong to C are enclosed in a circle.
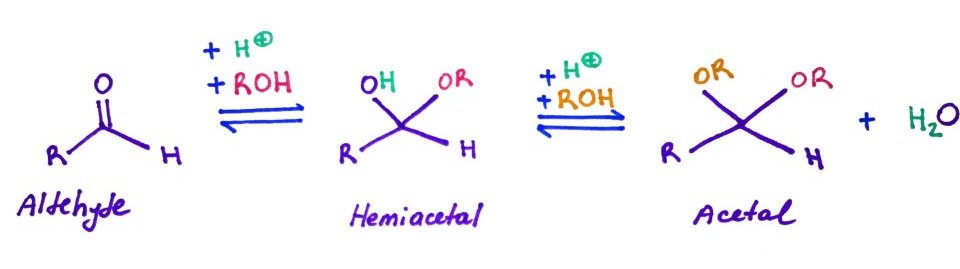
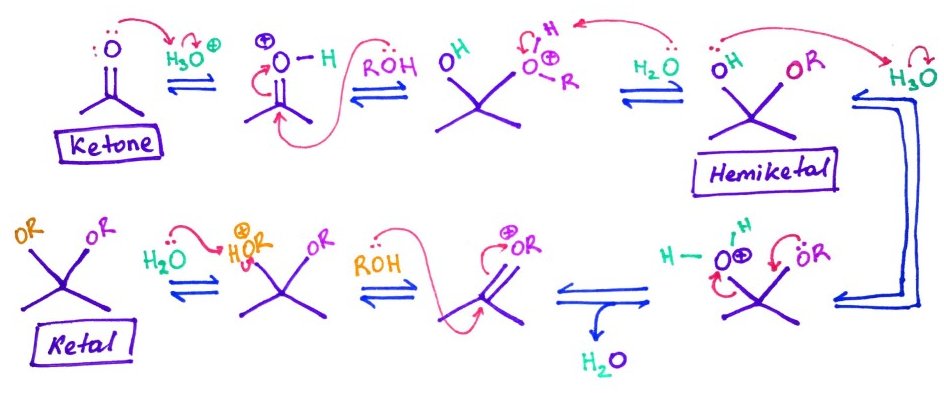
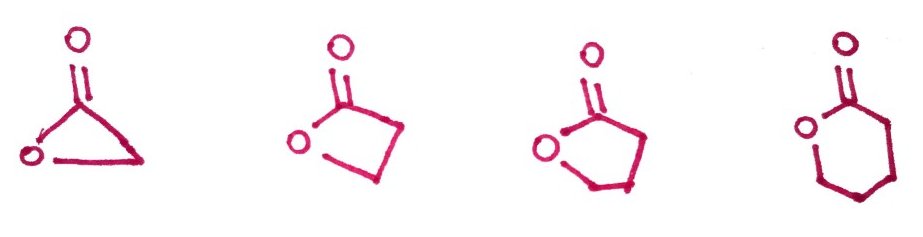
7.Lactones, Hemiacetals, Hemiketals, Acetals and Ketals
Lactones - are cyclic esters. We will comeback to them again in later chapters on carbohydrates.
Hemiacetals and hemiketals - are compounds that results from the addition of an alcohol to an aldehyde or a ketone respectively.
Acetals or ketals are formed when a second alcohol group has been added to the structure.